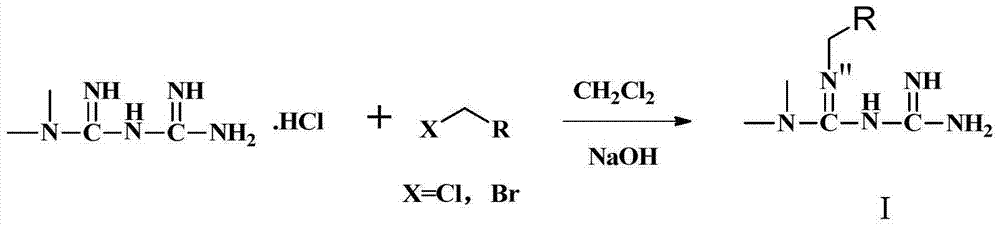
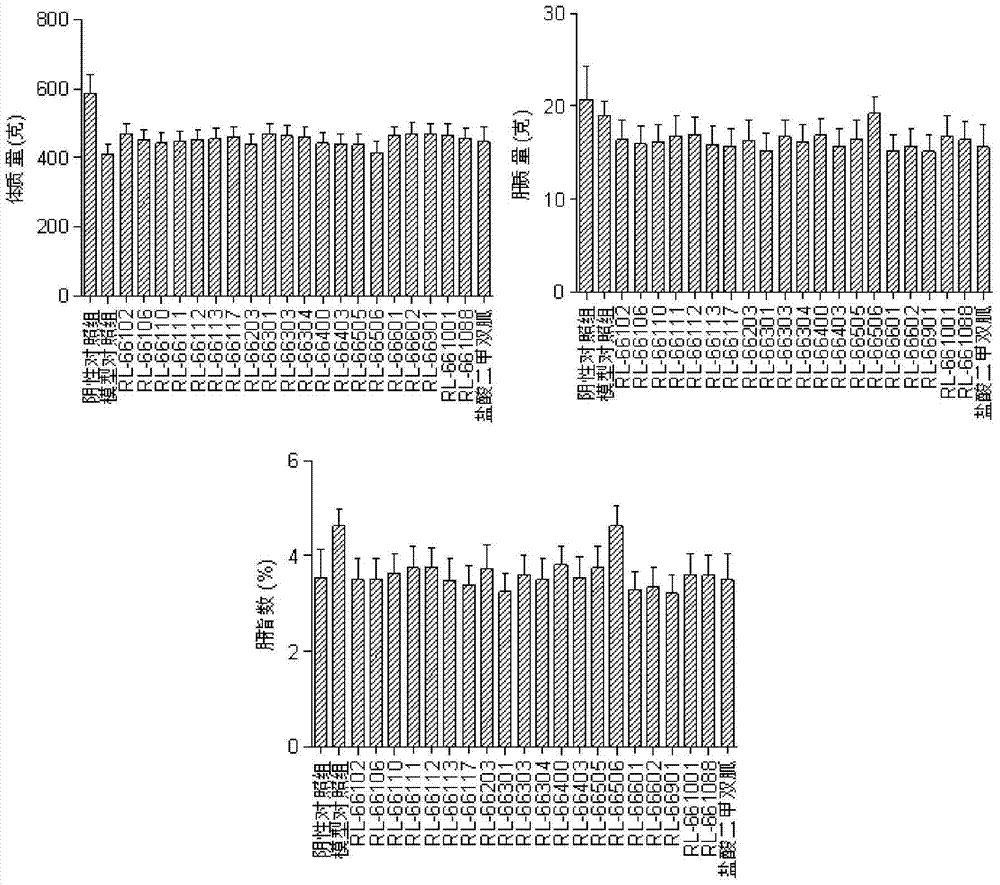
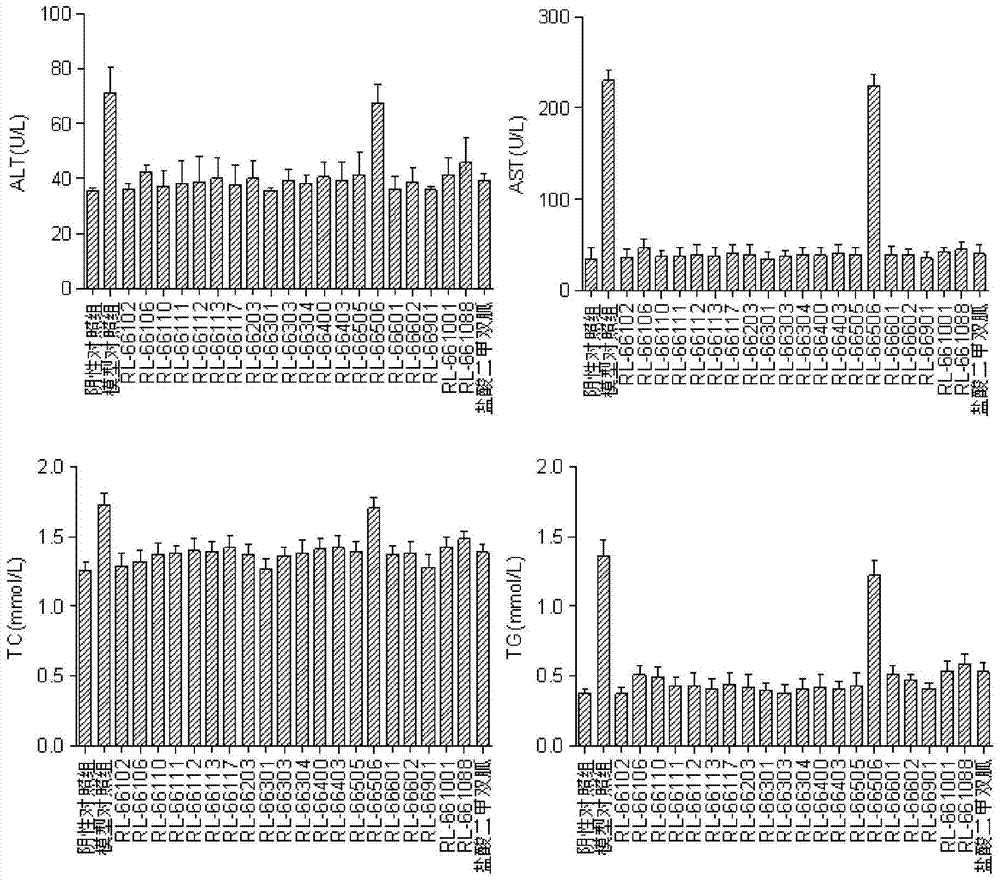
A kind of biguanide compound and its preparation method and application
A compound, biguanide technology, applied in the field of medicine, can solve the problem of lack of treatment methods and drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: Synthesis of RL-66102
[0102] In a 100ml round bottom bottle, add metformin hydrochloride 4g (24mmol), dichloromethane 50ml, add 25% sodium hydroxide 40ml, stir, after clarification, add potassium iodide 3 small grains, then add dropwise 3-bromopropene 2.9g / 2.1ml (24mmol), dripped in about 10 minutes, stirred magnetically, and ran the plate after 1 hour (developing agent: dichloromethane:methanol:formic acid=4.3:0.7:3dr). A total of 20 hours of reaction, post-processing. The reaction solution was transferred to a separatory funnel, and the dichloromethane and aqueous phase were separated. The aqueous phase was extracted three times with 20 ml of dichloromethane each time. The dichloromethane liquids were combined, and the dichloromethane was evaporated to dryness to obtain 2.3 g of crude oil. The crude product was subjected to column chromatography on 100-200 mesh silica gel (mobile phase: dichloromethane:methanol=900:100) to obtain 0.75 g of the crude...
Embodiment 2
[0103] Embodiment 2: Synthesis of RL-66106
[0104] In a 100ml round bottom bottle, add metformin hydrochloride 2g (12mmol), dichloromethane 25ml, ice bath, add 25% sodium hydroxide 10ml and stir, then add bromooctane 2.31g (12mmol), potassium iodide 3 pellets, magnetic Stirring, room temperature, a total of 30 hours of reaction, post-processing. The reaction solution was transferred to a separatory funnel, and the dichloromethane and aqueous phase were separated. The aqueous phase was extracted three times with 20 ml of dichloromethane each time. The dichloromethane liquids were combined, and the dichloromethane was evaporated to dryness to obtain 1.6 g of crude oil. The crude product was subjected to column chromatography on 100-200 mesh silica gel (mobile phase: dichloromethane:methanol=900:100) to obtain 0.95 g of the crude product of the target product. Reverse-phase separation was performed on a medium-pressure column, mobile phase: water / acetonitrile=98 / 2, and 0.45 g...
Embodiment 3
[0105] Embodiment 3: Synthesis of RL-66110
[0106] In a 100ml round bottom bottle, add metformin hydrochloride 2g (12mmol), dichloromethane 25ml, ice bath, add 25% sodium hydroxide 10ml, stir, after clarification, add benzyl bromide 2.05g (12mmol), potassium iodide 3 pellets, magnetic Stirring, room temperature, a total of 18 hours of reaction, post-processing. A solid was seen in the reaction flask, which was filtered, washed with 2 ml of dichloromethane, and dried to obtain 0.9 g of a colorless solid. The solid was heated and dissolved in 150 ml of acetonitrile, filtered, left to cool, and crystallized to obtain 0.42 g of a colorless solid. Yield 16.0%. The proton nuclear magnetic spectrum data of product are: 1 H NMR (600MHz, DMSO) δ7.29(d, J=7.5Hz, 2H), 7.23(t, J=7.6Hz, 2H), 7.11(t, J=7.3Hz, 1H), 5.21(s, 4H ), 4.17 (s, 2H), 2.78 (s, 6H). HRMS [M+H] 220.35.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com