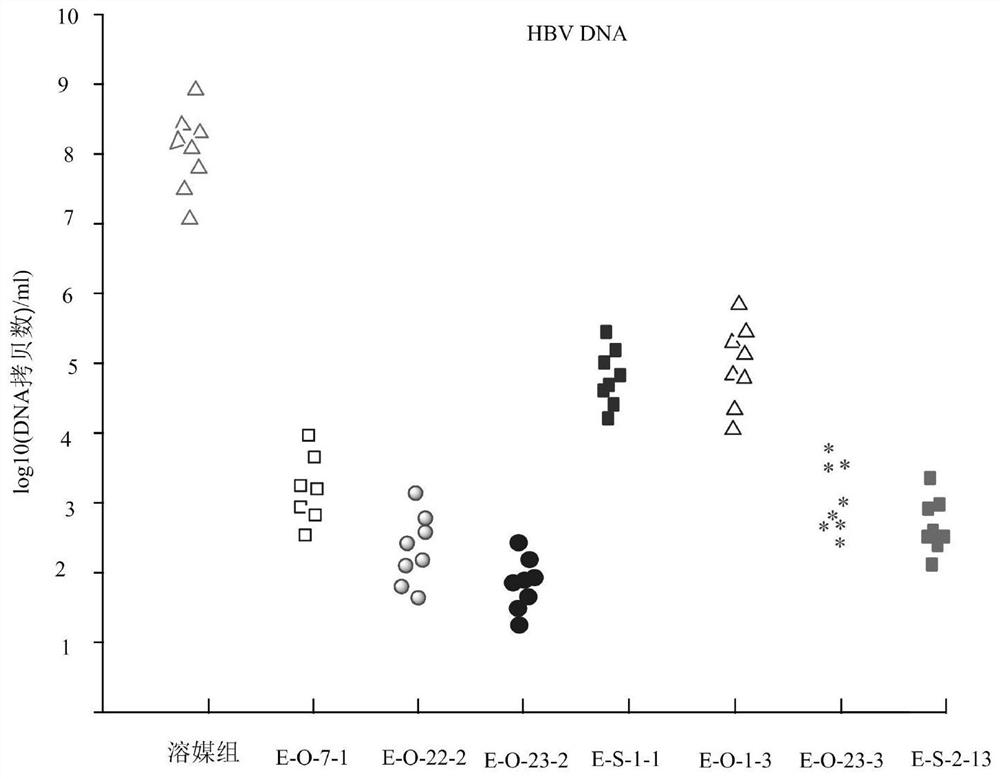
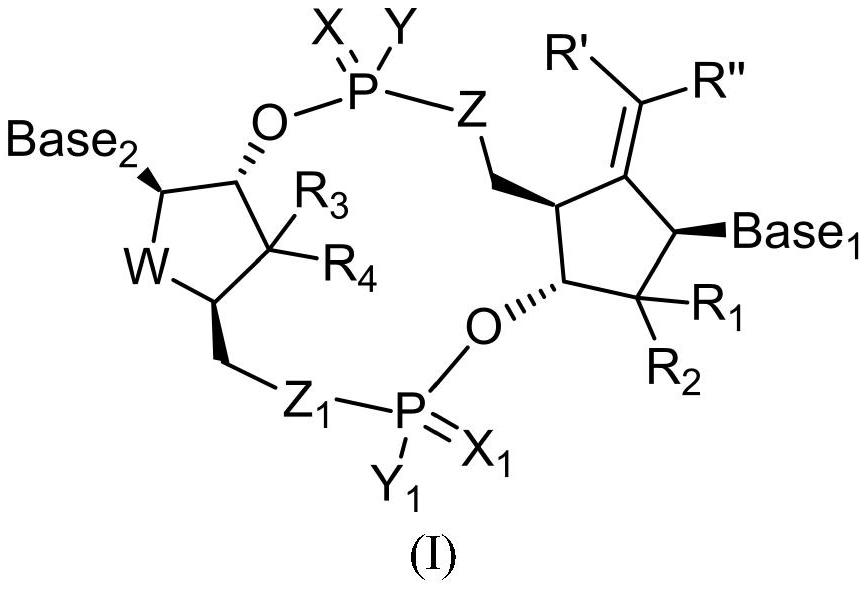
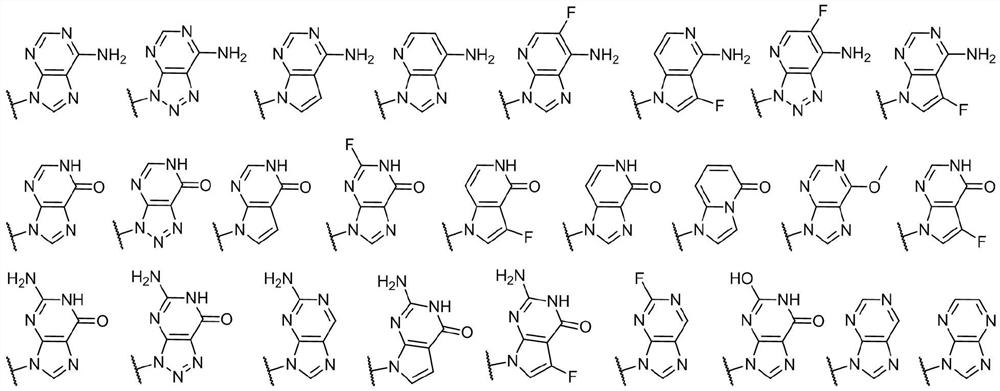
Cyclic dinucleotide compound, its preparation method and application
A compound, independent technology, applied in the field of nucleoside analogs, which can solve the problems of intractable effect, loss of activity, low permeability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0619]
[0620] The first step: (2R,3S,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-fluoro-2-(2-iso Butyrylamino-6-oxo-1H-purin-9(6H)-yl)tetrahydrofuran-3-yl(2-cyanoethyl)phosphonate (C1-1)
[0621] To (2R,3S,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-fluoro-2-(2-isobutyrylamino -6-Oxo-1H-purin-9(6H)-yl)tetrahydrofuran-3-yl(2-cyanoethyl)diisopropylphosphoramidite (3g, 3.5mmol) in ACN (15mL) Water (0.126 mL, 7 mmol) and 2,2,2-trifluoroacetic acid pyridinium salt (0.811 mg, 4.2 mmol) were added to the solution. The resulting mixture was stirred at room temperature and the progress of the reaction was monitored by LCMS / TLC. After the phosphoramidite was consumed, the reaction mixture containing the product was used in the next step without purification. LCMS (ES, m / z): 775.3 [M+H] +
[0622] The second step: (2R,3S,4R,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-fluoro-2-(2-iso Butyrylamino-6-oxo-1H-purin-9(6H)-yl)tetrahydrofuran-3-ylph...
Embodiment 2-29
[0641] Examples 2 to 29 were prepared according to a method similar to that described in Example 1 above, using the corresponding intermediates (protected nucleosides) in the table below to prepare (2-cyanoethyl)diisopropylphosphoramidites P2-i, (2-cyanoethyl)diisopropylphosphoramidite P1-i prepared from the corresponding methylenecyclopentyl nucleoside analog by oxidation, decyanoethylation, and deprotection Coupling, and then through oxidation, deprotection and ring closure, reoxidation, removal of all protecting groups, salt formation and other steps to obtain the final product;
[0642]
[0643]
[0644]
[0645]
Embodiment 30
[0647]
[0648] The first step: adenosine phosphoramidite P1-1 (17.7g, 20mmol) was dissolved in ACN (200mL) solution. Water (0.72 mL, 40 mmol) and pyridine·TFA (4.64 g, 24 mmol) were added to the solution, and the resulting mixture was stirred at room temperature for 25 minutes. Tert-butylamine was then added, the solution was stirred for 15 minutes, and the solvent was removed in vacuo. Dissolve the residue in 3% DCA in CH 2 Cl 2 A mixture of the solution (150 mL) and water (3.6 mL) was stirred for 20 minutes. The reaction was quenched with MeOH and pyridine. The solvent was removed by concentration under reduced pressure, and the residue was purified by silica gel column chromatography using CH 2 Cl 2 / EtOH / MeOH (80 / 15 / 5) as eluent afforded C2-1 (7.15 g; 80% yield). LC-MS: tR=4.92min, m / z=448[M+H] + , m / z=446[M-H] - .
[0649] The second step: in the molecular sieve In the presence of the compound inosine phosphoramidite (7.72g, 10mmol) was added to C2-1 (2.24g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com