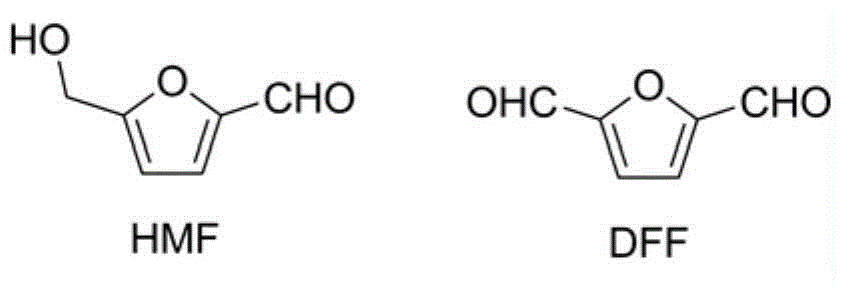
Method for separating 5-hydroxymethyl furfural and 2,5-diformylfuran mixture
A technology of diformylfuran and hydroxymethylfurfural, applied in the direction of organic chemistry, can solve problems such as difficult to achieve effective separation, and achieve the effects of easy scale-up, pollution reduction, and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
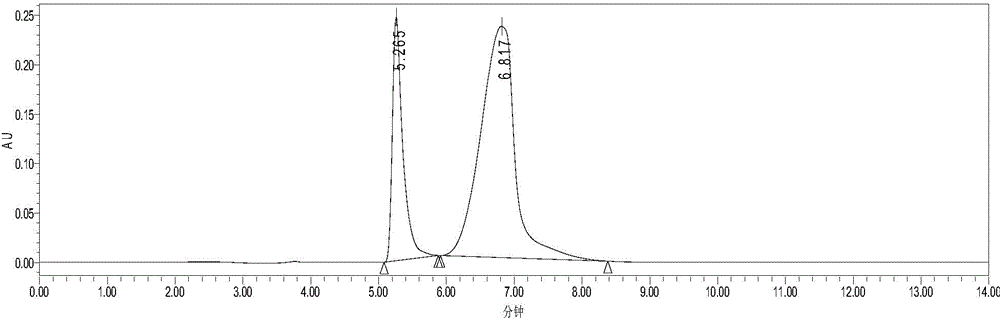
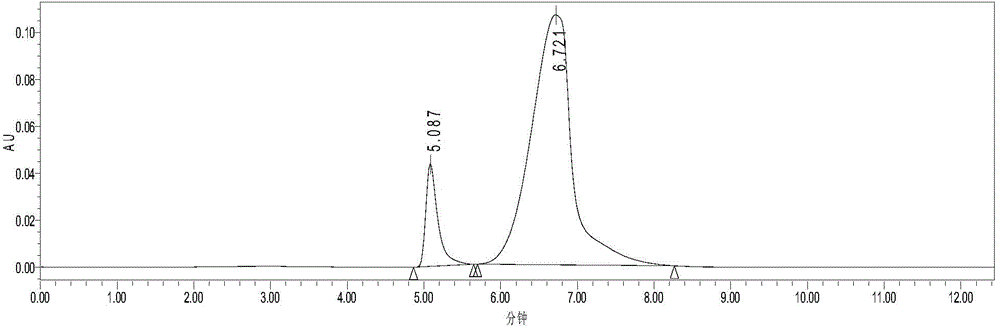
[0025] The mixture of HMF (content 24%) and DFF (content 76%) was dissolved in ethyl acetate so that the concentrations were 0.67mg / mL and 2.07mg / mL respectively (see figure 1 , the retention times of HMF and DFF are 5.27min and 6.82min respectively), add 2 times the volume of DES (choline chloride: glycerol=1:2, mol / mol), stir at room temperature and 400r / min for 10min, divide Phase, liquid chromatography analysis showed that HMF and DFF concentrations in ethyl acetate were 0.12mg / mL and 1.00mg / mL (see figure 2 ), remove ethyl acetate under reduced pressure to obtain DFF with a purity of 89%.
Embodiment 2
[0027] The mixture of HMF (content 24%) and DFF (content 76%) was dissolved in ethyl acetate so that the concentrations were 0.67mg / mL and 2.07mg / mL respectively, and 5 times the volume of DES (choline chloride: glycerol =1:2, mol / mol), stirred at room temperature and 400r / min for 10min, phase separation, liquid chromatography analysis showed that the concentrations of HMF and DFF in ethyl acetate were 0.07mg / mL and 0.84mg / mL respectively, and the Ethyl acetate was removed under reduced pressure to obtain DFF with a purity of 93%.
Embodiment 3
[0029] The mixture of HMF (content 24%) and DFF (content 76%) was dissolved in ethyl acetate so that the concentrations were 0.67mg / mL and 2.07mg / mL respectively, and 1 volume of DES (choline chloride: urea =1:2, mol / mol), stirred at room temperature and 400r / min for 10min, separated phases, and liquid chromatography analysis showed that the concentrations of HMF and DFF in ethyl acetate were 0.34mg / mL and 1.97mg / mL respectively. Ethyl acetate was removed under reduced pressure to obtain DFF with a purity of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com