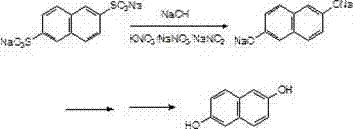
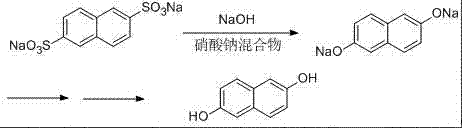
Synthetic method of 2,6 dihydroxy naphthlene
A technology of dihydroxynaphthalene and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of uneven mixing of materials, difficult separation, and high consumption of alkali, and achieves convenient synthesis steps, It is not easy to separate and the effect of uniform reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 166g of sodium 2,6-naphthalene disulfonate, 100g of solid sodium hydroxide, 166g of potassium nitrate, sodium nitrite and a mixture of sodium nitrate (88g of potassium nitrate, 66g of sodium nitrite, and 12g of sodium nitrate) into the alkali melting pot , then slowly heat up to 150°C, stir, continue to heat up to 320°C, and keep the alkali fusion at 320°C-325°C for 3 hours. Then lower the temperature to 200°C, slowly add 450g of water dropwise to dilute, and extract 2,6-sodium naphthalenediol with 700mL trioctane tertiary amine solvent.
[0025] The extracted sodium 2,6-naphthalenediol was acidified with sulfuric acid, filtered, and the filter cake was refined with 300 mL of ethanol-water mixed solvent to obtain 2,6-dihydroxynaphthalene, the purity of which was analyzed by liquid chromatography was above 99%, and the yield was 75%. %.
Embodiment 2
[0027] Put 166g2,6-sodium naphthalene disulfonate, 115g solid sodium hydroxide, a mixture of 182g potassium nitrate, sodium nitrite and sodium nitrate (wherein potassium nitrate 92g, sodium nitrite 76g, sodium nitrate 14g) in the alkali melting pot, Then slowly raise the temperature to 150°C, stir, continue to heat up to 310°C, and keep it at 310°C-320°C for alkali fusion for 3.5 hours; then cool down to 200°C, slowly add 450g of water to dilute, and use 700mL trioctane tertiary amine Solvent extraction of sodium 2,6-naphthalene diphenol;
[0028] The extracted sodium 2,6-naphthalene diphenol was acidified with sulfuric acid, filtered, and the filter cake was refined with 300 mL of ethanol-water mixed solvent to obtain 2,6-dihydroxynaphthalene, the purity of which was analyzed by liquid chromatography was over 99%, and the yield was 76% %.
Embodiment 3
[0030] Put 166g of sodium 2,6-naphthalene disulfonate, 83g of solid sodium hydroxide, 135g of potassium nitrate, sodium nitrite and a mixture of sodium nitrate into the alkali melting pot (65g of potassium nitrate, 60g of sodium nitrite, and 10g of sodium nitrate) , then slowly raise the temperature to 150°C, stir, continue to heat up to 320°C, and keep it at 320°C-330°C for alkali fusion for 2.5 hours; then cool down to 200°C, slowly add 450g of water to explain, and use 700mL of Amine solvent extraction of sodium 2,6-naphthalenediol.
[0031] The extracted sodium 2,6-naphthalenediol was acidified with sulfuric acid, filtered, and the filter cake was refined with 300 mL of ethanol-water mixed solvent to obtain 2,6-dihydroxynaphthalene, the purity of which was analyzed by liquid chromatography was above 99%, and the yield was 77% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com