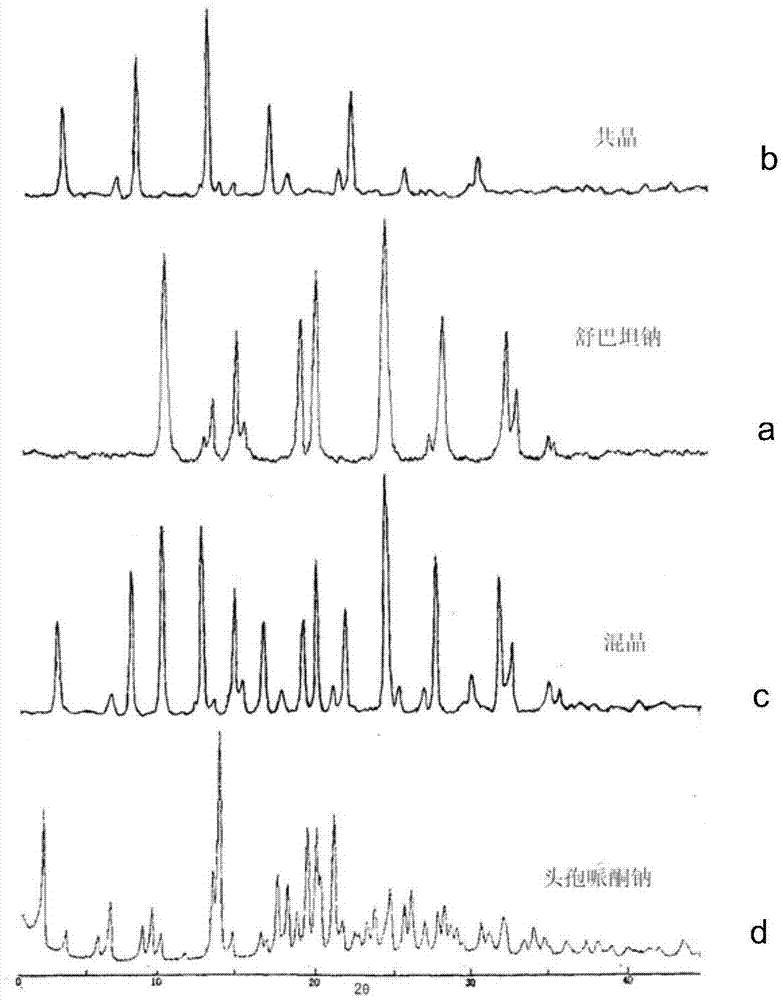
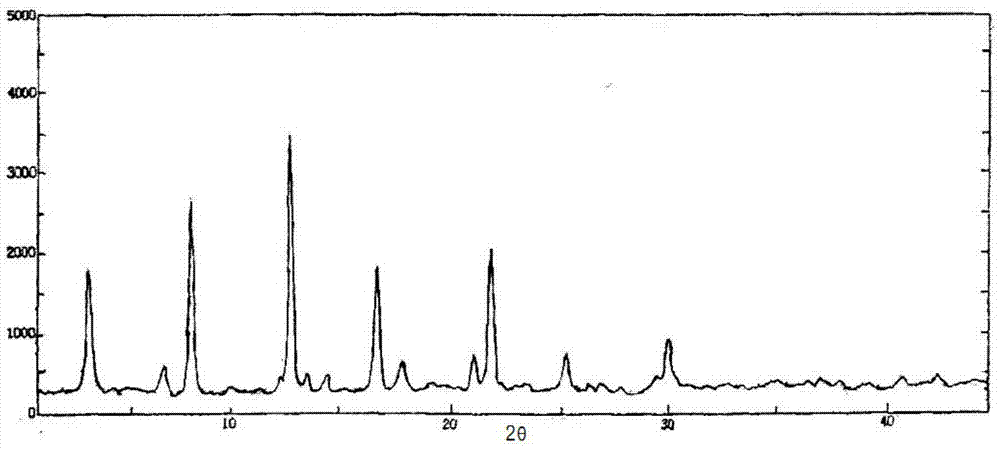
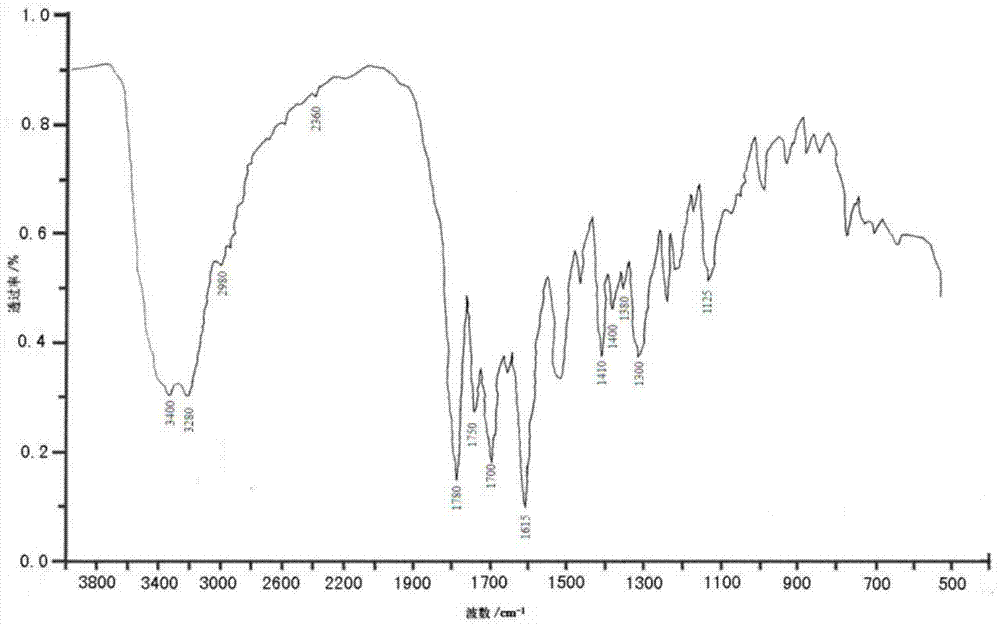
Cefoperazone sodium-sulbactam sodium eutectic crystal and composition, and preparation methods thereof
A technology of cefoperazone sodium and sulbactam sodium, applied in the direction of active ingredients of heterocyclic compounds, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of short validity period and poor stability, and achieve low content of related substances and crystal form Concentrated particle size distribution, reducing the risk of adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of cefoperazone sodium and sulbactam sodium cocrystal:
[0037] Add cefoperazone sodium (13.35g, 0.02mol) and sulbactam sodium (5.10g, 0.02mol) to a volume ratio of 6.5:3:0.5 water:ethanol:acetone solution that is 10 times the weight of cefoperazone sodium and sulbactam sodium During the process, control the temperature at 10-20°C and ultrasonically dissolve it; after the dissolution is clear, add tetrahydrofuran whose volume is 1 times the weight of cefoperazone sodium and sulbactam sodium at a constant speed under stirring at 15-20rmp, and control the dropping time for 10-15 minutes; After finishing, stir and lower the temperature, the stirring and cooling is stirring at a rotating speed of 25-30rmp for 5 minutes to cool down to 0-10°C, then stirring at a rotating speed of 10-12rmp for 5 minutes to cool down to -5-0°C, and standing for 20-30 hours , filtered, washed twice with ethanol:tetrahydrofuran solution with a volume ratio of 5:5, 0.2 t...
Embodiment 2
[0060] Embodiment 2: the preparation of cefoperazone sodium and sulbactam sodium cocrystal:
[0061] Add cefoperazone sodium (26.7g, 0.04mol) and sulbactam sodium (10.2g, 0.04mol) to a volume ratio of 6.5:3:0.5 water:ethanol:acetone solution that is 20 times the weight of cefoperazone sodium and sulbactam sodium In the process, control the temperature at 10-20°C and ultrasonically dissolve it; after the dissolution is clear, add tetrahydrofuran whose volume is twice the weight of cefoperazone sodium and sulbactam sodium at a constant speed under stirring at 15-20rmp, and control the dropping time for 10-15 minutes; After finishing, stir and lower the temperature, the stirring and cooling is stirring at a rotating speed of 25-30rmp for 5 minutes to cool down to 0-10°C, then stirring at a rotating speed of 10-12rmp for 5 minutes to cool down to -5-0°C, and standing for 20-30 hours , filtered, washed 2 times with a volume ratio of 5:5 ethanol: tetrahydrofuran solution, each 0.5 t...
Embodiment 3
[0062] Embodiment 3: Preparation of cefoperazone sodium and sulbactam sodium co-crystal and mixed crystal of sulbactam sodium:
[0063] Add cefoperazone sodium (26.7g, 0.04mol) and sulbactam sodium (12.8g, 0.055mol) to a volume ratio of 6.5:3:0.5 water:ethanol:acetone solution that is 15 times the weight of cefoperazone sodium and sulbactam sodium During the process, control the temperature at 10-20°C and ultrasonically dissolve it; after the dissolution is clear, add tetrahydrofuran whose volume is 1.5 times the weight of cefoperazone sodium and sulbactam sodium at a constant speed under stirring at 15-20rmp, and control the dropping time for 10-15 minutes; Complete, stirring and cooling down, the stirring and cooling is cooling down to 0-10°C for 5 minutes at a rotating speed of 25-30rmp, then cooling down to -5-0°C for 5 minutes under stirring at a rotating speed of 10-12rmp, and standing for 20-30 hours , filtered, washed twice with ethanol:tetrahydrofuran solution with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com