Duplex PCR detection kit and detection method for Listeria monocytogenes and Enterococcus faecium
A technology for Listeria and Enterococcus faecium, which is applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection to achieve the effects of convenient identification, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1. Design and screening of primers of the present invention
[0019] According to the known full-length genome sequences of Listeria monocytogenes and Enterococcus faecium, specific genes were screened for primer design, and the hlyA gene of Listeria monocytogenes and the ddl gene of Enterococcus faecium were finally selected, and their specificity was designed and screened. Primers (Table 1), marked as LM-F (SEQ ID NO: 1), LM-R (SEQ ID NO: 2), EFM-F (SEQ ID NO: 3), EFM-R (SEQ ID NO :4).
[0020] Table 1 Primer information
[0021]
Embodiment 2
[0022] Example 2. Establishment and optimization of screening double PCR reaction system
[0023] 2.1 Genome Extraction
[0024] The clinical isolates of Enterococcus faecium and the clinical isolates of Listeria monocytogenes (preserved in our laboratory) were inoculated on nutrient agar medium for pure culture, and then a single colony was picked and cultured in the corresponding enrichment solution until logarithmic growth In the later stage, 2 mL of the bacterial liquid was centrifuged at 10,000 rpm for 1 min to collect the bacterial cells, and the genomic DNA of the two bacterial strains were extracted with a bacterial DNA extraction kit, and stored at -20°C for future use.
[0025] 2.2 Establishment and optimization of multiplex PCR reaction system
[0026] A 25 μL PCR reaction system was used for detection, including: 12.5 μL of 2x Es Taq MasterMix, 2 μL of DNA template, 0.25 μL of each primer, and sterilized deionized water to make up to 25 μL. The reaction condition...
Embodiment 3
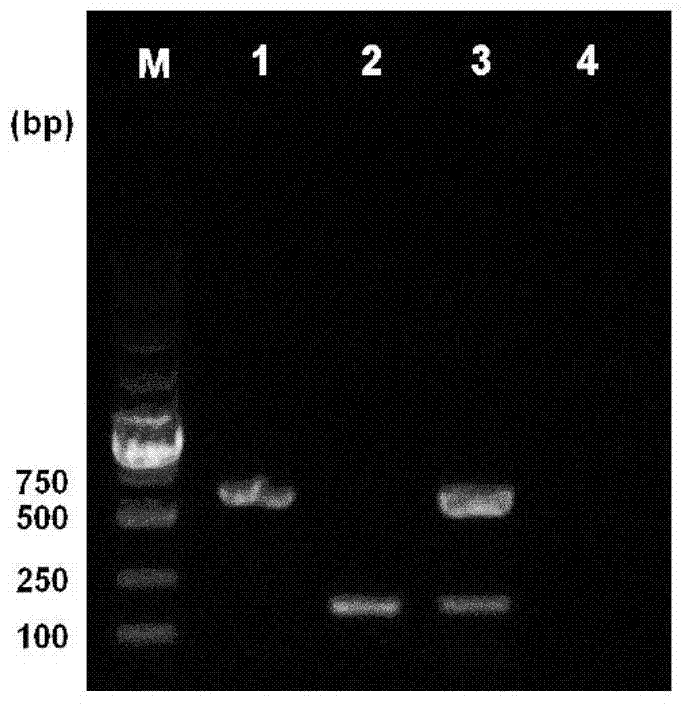
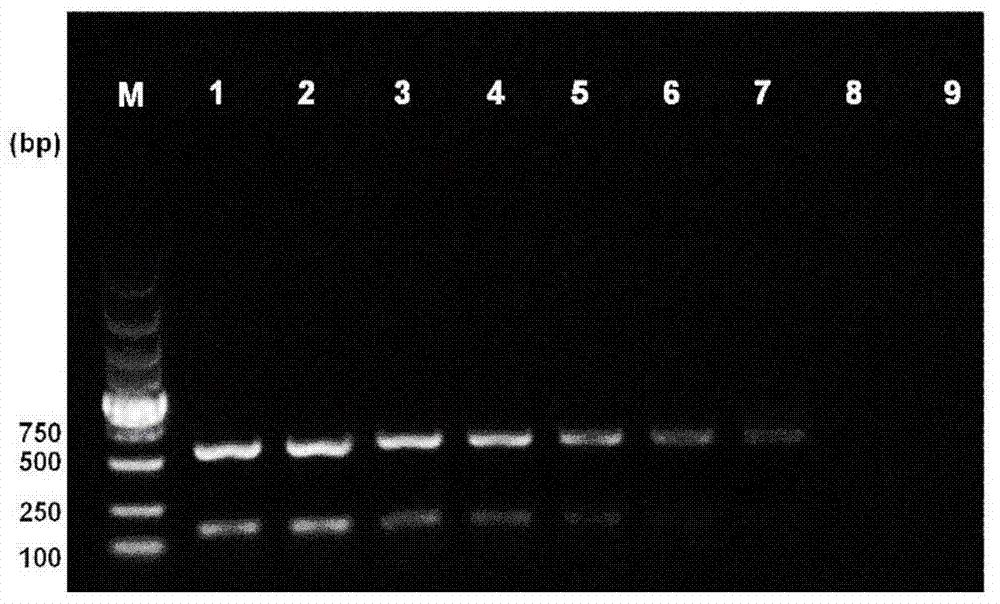
[0027] Embodiment 3. The specificity and the sensitivity of double PCR reaction system
[0028] 3.1 Specificity of duplex PCR
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com