Novel drug eluting balloon catheter
A balloon catheter and drug technology, applied in balloon catheters, drug devices, other medical devices, etc., can solve the problems of difficult solution retention, damage to coating integrity, complicated production process, etc., and achieve low hydrophilicity, Good lipophilic and hydrophilic properties, the effect of reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
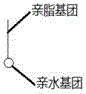
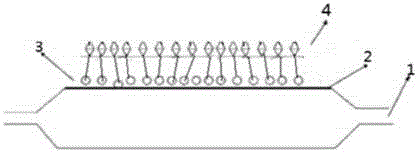
[0039] Such as figure 2 As shown, firstly, the surface of the balloon is pretreated by plasma cleaning technology, and the debris on the surface of the balloon is cleaned by using plasma cleaning technology. The plasma cleaning preferably uses an inert gas, such as argon, with a power of 50KHz. The pressure is 0.1atmatm, and the cleaning time is 10min. Then use polyethylene glycol with good hydrophilicity and lipophilicity as the bottom layer material (such as figure 1 As shown, the polymer material has both hydrophilic groups and lipophilic groups), then spray the polyethylene glycol bottom layer with a molecular weight of 70K, with a thickness of 1 μm, and then spray a layer of polyethylene glycol with a molecular weight of 15K and paclitaxel mixed Drug-loaded coating with a drug loading of 2.5 μg / mm 2 , with a thickness of 10 μm; after the coating is dry, it is finally folded.
Embodiment 2
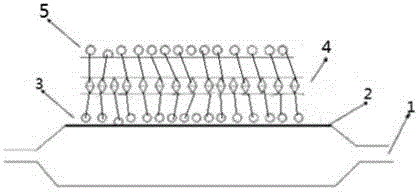
[0041] Such as image 3 As shown, firstly, the surface of the balloon is pretreated by plasma cleaning technology, and the impurities on the surface of the balloon are cleaned by using plasma cleaning technology. The plasma cleaning preferably uses an inert gas, such as neon gas, with a power of 10KHz. The pressure is 0.01atm, and the cleaning time is 5min. Then take the hydrophilic and lipophilic polystearate as the bottom material (such as figure 1 As shown, the polymer material has both hydrophilic groups and lipophilic groups), spraying one deck of molecular weight is 51K polystearic acid ester bottom layer, thickness 2 μ m, then spraying one layer of molecular weight is 5K hardic acid ester and Paclitaxel-mixed drug-loaded coating with a drug loading of 1 μg / mm 2 , with a thickness of 50 μm; after the coating is dried, folded, wound and then sprayed with a surface layer of polyglycerol ester with a molecular weight of 2K, the thickness is 2 μm.
Embodiment 3
[0043] Such as image 3 As shown, firstly, the surface of the balloon is pretreated by plasma cleaning technology, and the impurities on the surface of the balloon are cleaned by using plasma cleaning technology. Preferably, the plasma cleaning uses an inert gas, such as argon, with a power of 50KHz. The pressure is 0.1atm, and the cleaning time is 10min. Then use lipophilic and hydrophilic dextran as the bottom material (such as figure 1 As shown, the polymer material has both hydrophilic groups and lipophilic groups), spraying a layer of dextran bottom layer with a molecular weight of 150K, with a thickness of 0.1 μm, and then spraying a layer of dextran and rapamycin with a molecular weight of 39K Drug-loaded coating mixed with prime, with a drug loading of 2.5 μg / mm 2 , with a thickness of 1 μm; after the coating is dried, folded, wound up and then sprayed with a layer of polycitrate with a molecular weight of 39K, the thickness is 0.1 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com