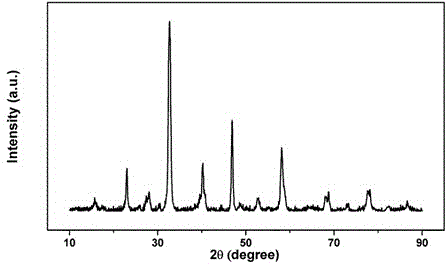
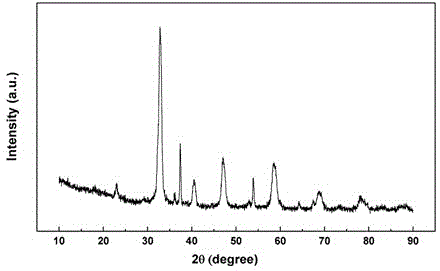
Low temperature preparation method of layered perovskite
A perovskite, layered technology, applied in the field of layered perovskite preparation, can solve the problems of unfavorable wide application, harsh preparation conditions, low specific surface area, etc., and achieves low cost, low preparation temperature and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 6.50 g La(NO 3 ) 3 ·6H 2 O (15 mmol), 2.68 g Mn(NO 3 ) 2 solution (50 wt%, 7.5 mmol), and 0.50 g MnCl 2 (2.5 mmol) was dissolved in water to form solution A; 6.33 g citric acid (30 mmol) was mixed with 2.25 g NH 4 HCO 3 (28.5 mmol) was dissolved in water to form solution B. Mix A and B solutions, stir evenly, distill under reduced pressure, lose water to form a thick jelly, then dry and roast at 700 °C for 5 h to obtain a blue-black precursor powder. Weigh 1.00 g of precursor powder, containing 0.10 mol L -1 NH 4 HCO 3 solution in an autoclave, reacted at 80 °C for 12 h, separated by suction filtration, washed with deionized water and ethanol, dried, and then calcined at 800 °C for 2 h to obtain blue-black layered perovskite La 3 mn 2 o 7+δ powder.
Embodiment 2
[0022] 2.17 g La(NO 3 ) 3 ·6H 2 O (5 mmol), 2.39 g Ca(NO 3 ) 2 4H 2O (10 mmol), 2.68 g Mn(NO 3 ) 2 solution (50 wt%, 7.5 mmol), and 0.50 g MnCl 2 (2.5 mmol) was dissolved in water to form solution A; 6.33 g citric acid (30 mmol) was mixed with 2.25 g NH 4 HCO 3 (28.5 mmol) was dissolved in water to form solution B. Mix A and B solutions, stir evenly, distill under reduced pressure, lose water to form a thick jelly, dry, and then roast at 800 °C for 5 h to obtain the precursor powder. Weigh 1.00 g of precursor powder, containing 0.10 mol L -1 NH 4 HCO 3 solution in an autoclave, reacted at 120 °C for 12 h, separated by suction filtration, washed with deionized water and ethanol, dried, and then roasted at 800 °C for 2 h to obtain the layered perovskite LaCa 2 mn 2 o 7 powder.
Embodiment 3
[0024] 1.73 g La(NO 3 ) 3 ·6H 2 O (4 mmol), 0.53 g Ba(NO 3 ) 2 and 1.17 g Co(NO 3 ) 2 ·6H 2 O (4 mmol) was dissolved in water to form solution A; 2.53 g of citric acid (12 mmol) and 0.26 g of p-chloroaniline (2 mmol) were dissolved in water to form solution B. Mix A and B solutions, stir evenly, distill under reduced pressure, lose water to form a thick jelly, dry, and then roast at 1000 °C for 2 h to obtain the precursor powder. Weigh 1.00 g of precursor powder, containing 0.10 mol L -1 NH 4 HCO 3 solution in an autoclave at 120 °C for 8 h, then separated by suction filtration, washed with deionized water and ethanol, dried, and then calcined at 700 °C for 2 h to obtain a blue-black layered perovskite La 2 BaCo 2 o 7 powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com