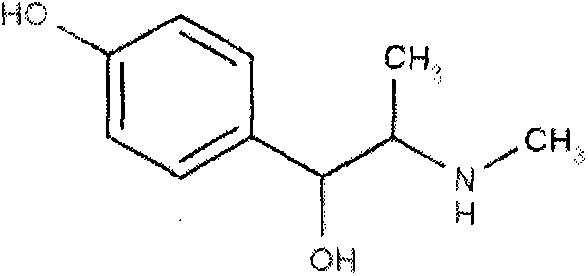
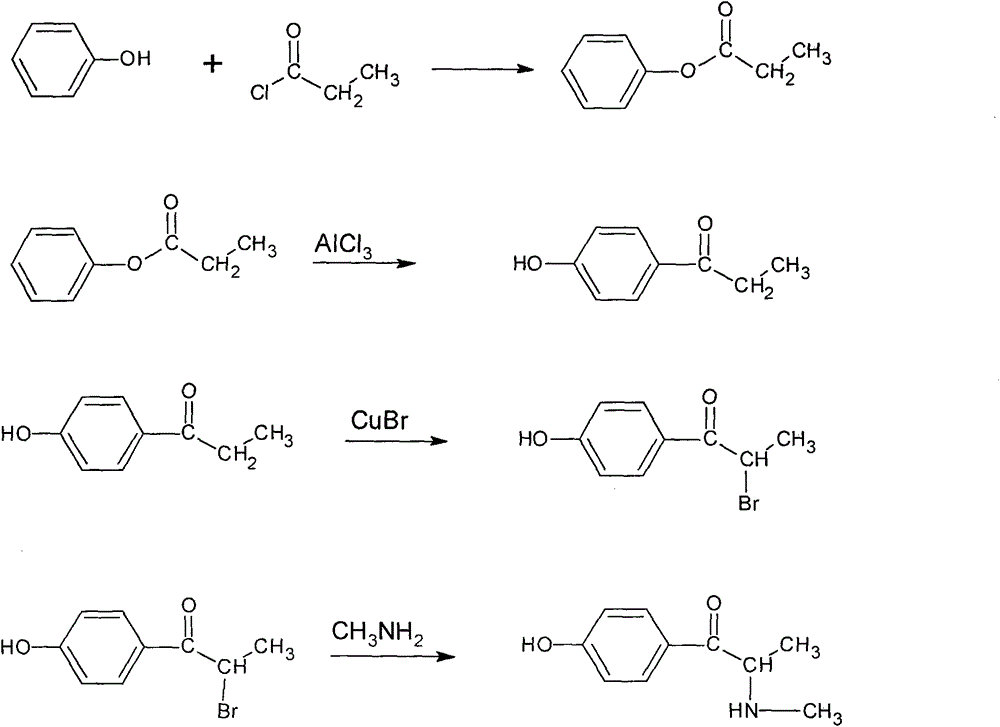
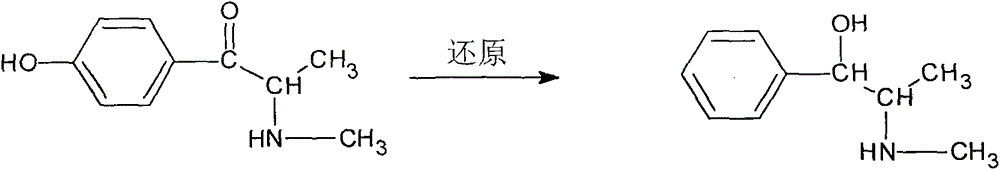
Synthetic method for methyl synephrine
A technology of methyl synephrine and synthetic method, applied in the field of synthesis of methyl synephrine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The synthetic route of the present invention is as follows:
[0015]
[0016]
[0017] 1. Preparation of phenol propionate Add 100g of phenol, 240mL of morpholine, and 130mL of benzene into a 1000mL four-necked round-bottomed flask equipped with a mechanical stirrer, a thermometer, a drying tube and a reflux condenser, and slowly add in an ice-salt bath Add 230g of propionyl chloride within 30min, raise to room temperature, continue to react to form white floc, continue to react for 1-3h, detect with thin-layer chromatography, concentrate the reaction solution, add water at 5-10℃ and stir well, static Set the layers, collect the organic phase, the water layer contains propionic acid, morpholine salt and propionyl chloride produced by the reaction, extract 2-4 times with an equal volume of benzene, combine the benzene liquid, and recover the benzene solvent under reduced pressure to obtain a colorless and transparent Phenol propionate.
[0018] 2. Preparation of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com