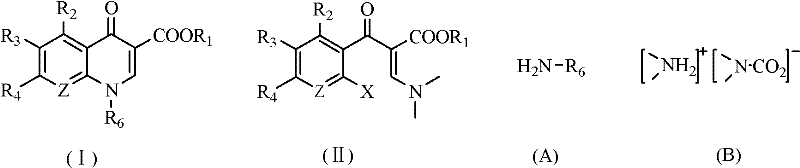
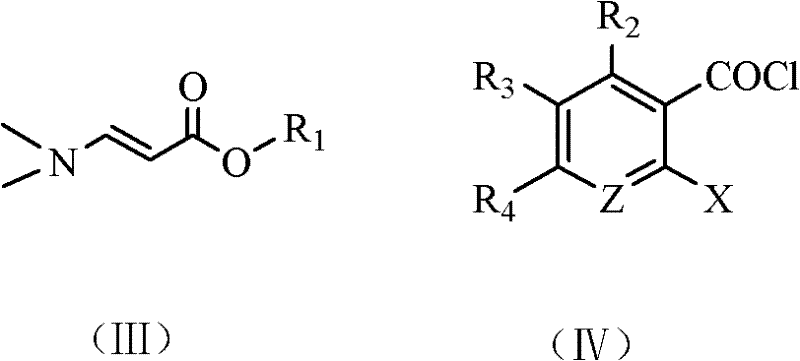
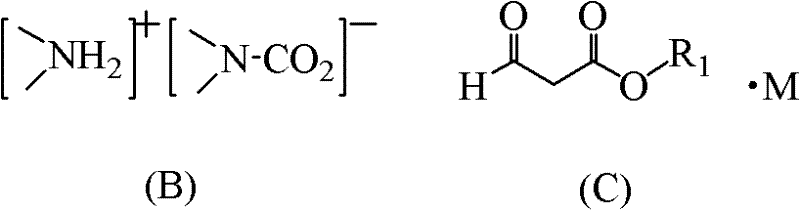Method for synthesizing quinolone main cycle compound
A technology of a cyclic compound and a synthesis method is applied in the field of key intermediates of antibacterial quinolones, and can solve the problems of low purity, incomplete reaction, difficulty in handling dimethylamine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Preparation of ethyl formyl acetate sodium salt: In a reaction kettle equipped with a stirrer, add 22g of ethyl acetate, 38g of ethyl formate, 200mL of toluene and 17g of sodium ethoxide in sequence, and slowly raise the temperature to 70°C under stirring, while the pressure Rising to 8 atm, the reaction was completed after 6 hours, and a mixed solution containing ethyl formylacetate sodium salt was prepared, which was cooled to 0°C for later use, wherein the amount of ethyl formylacetate sodium salt was 0.23 mol.
[0068] Add 15.4g of dimethylamine complex and 150mL of toluene into the reaction flask, cool in an ice-salt bath to 0-5°C, then add dropwise the above mixed solution containing ethyl formyl acetate sodium salt, stir the reaction for 40 minutes, stop, After the reaction solution was washed with water, the organic layer was distilled, and 30.0 g of a fraction at 118-121°C (7.5 mmHg) was taken, namely N,N-ethyl dimethylaminoacrylate, with a yield of 91.2%.
[0...
Embodiment 2
[0073] Preparation of ethyl formyl acetate sodium salt: In a reaction kettle equipped with a stirrer, add 22g of ethyl acetate, 38g of ethyl formate, 200mL of toluene and 17g of sodium ethoxide in sequence, and slowly raise the temperature to 70°C under stirring, while the pressure Rising to 8 atm, the reaction was completed after 6 hours, and a mixed solution containing ethyl formylacetate sodium salt was prepared, which was cooled to 0°C for later use, wherein the amount of ethyl formylacetate sodium salt was 0.23 mol.
[0074] Add 15.4g of dimethylamine complex and 150mL of toluene into the reaction flask, cool in an ice-salt bath to 0-5°C, then add dropwise the above mixed solution containing ethyl formyl acetate sodium salt, stir the reaction for 40 minutes, stop, After the reaction solution was washed with water, the organic layer was distilled, and 30.0 g of a fraction at 118-121°C (7.5 mmHg) was taken, namely N,N-ethyl dimethylaminoacrylate, with a yield of 91.2%.
[0...
Embodiment 3
[0079] One of the raw materials, 2,4,5-trifluoro-3-methoxybenzoyl chloride, was changed to 45.5g 2,4-dichloro-5-fluorobenzoyl chloride, and other conditions were the same as in Example 1 to obtain 52.6g The main ring compound of ciprofloxacin, i.e. 1-cyclopropyl-6-fluoro-7-chloro-1,4-dihydro-4-oxoquinolinyl-3-carboxylic acid ethyl ester, the yield is 85.0 %.
[0080] 1 MR(CDCl 3 )δ: 1.2 ~ 1.6 (4H, m, CH 2 ), 1.43 (3H, m, -OCH 2 CH 3 ), 3.53 (1H, m), 4.46 (2H, m, -OCH 2 CH 3 ), 8.07 (1H, d, ArH), 8.15 (1H, d, ArH), 8.54 (1H, s, CH=C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com