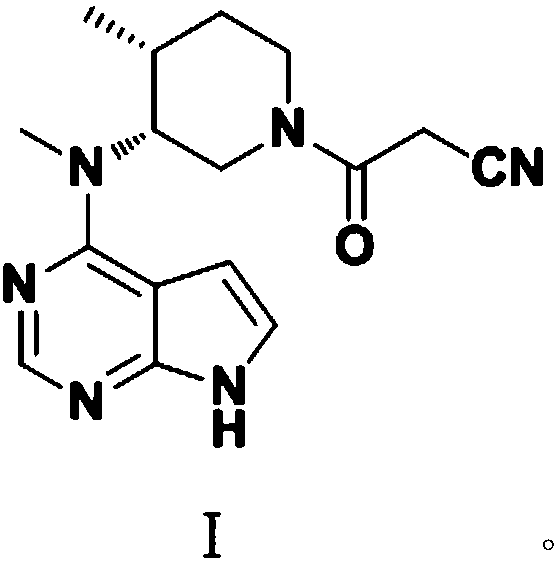
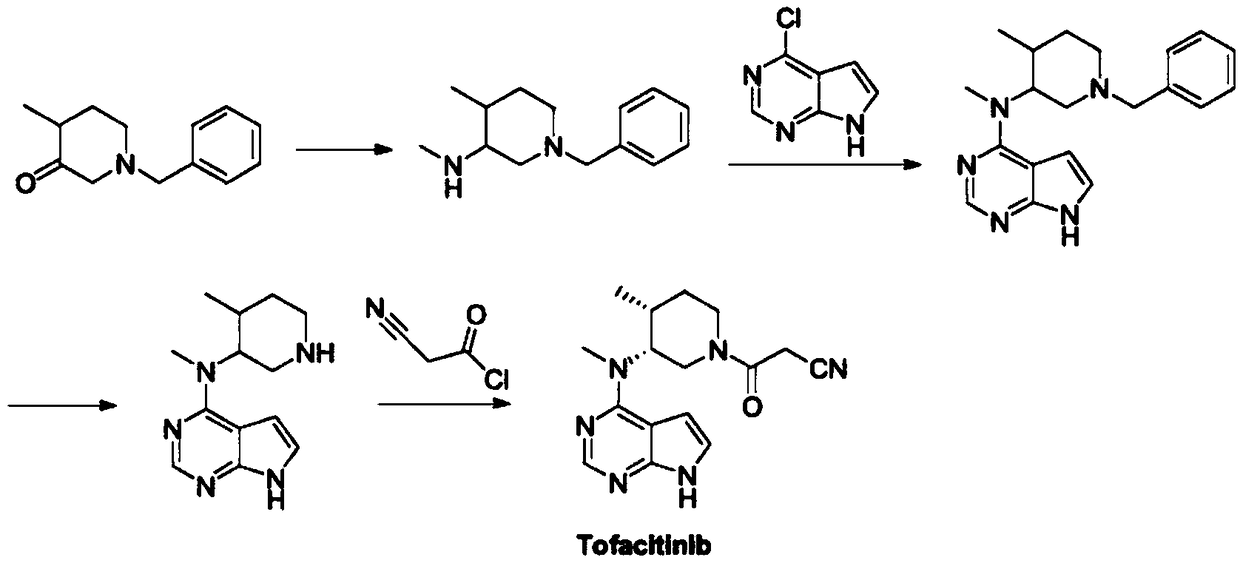
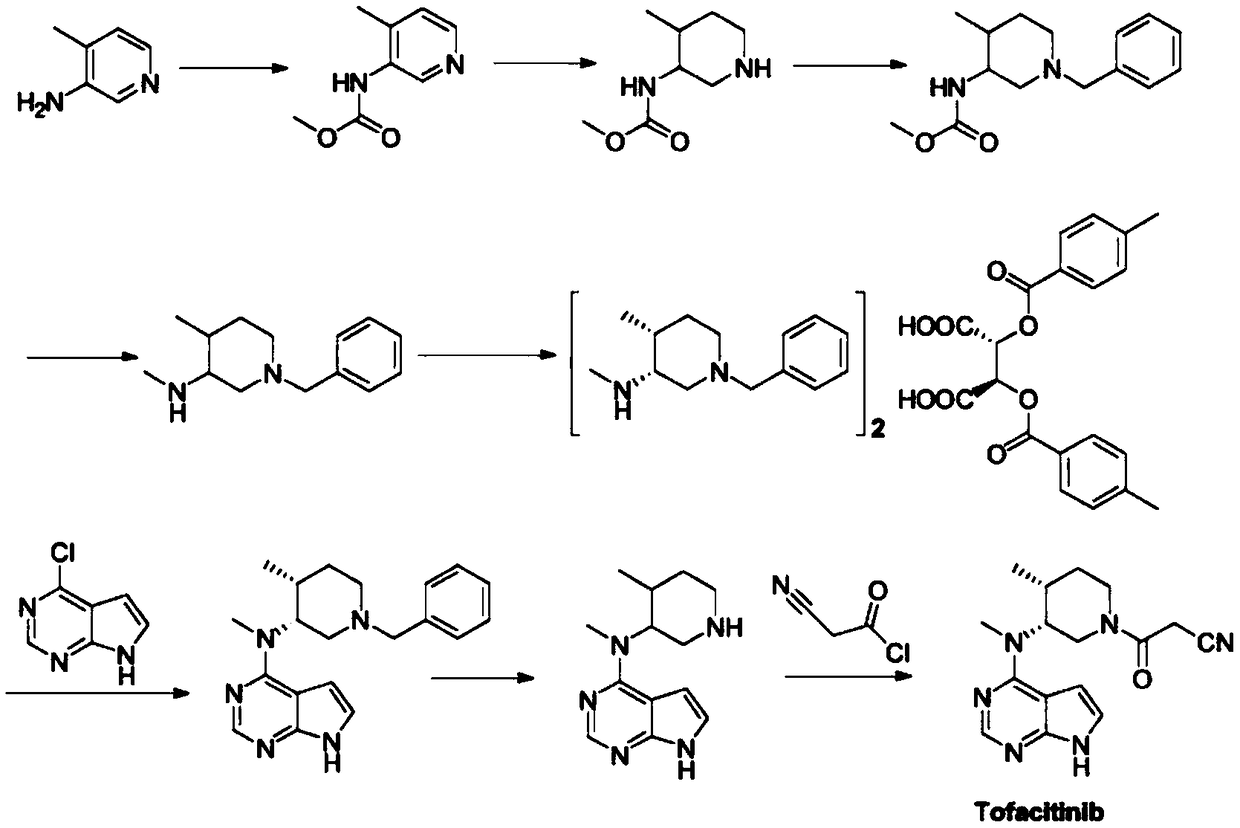
Synthetic method of tofacitinib
A synthetic method, the technology of tofacitinib, applied in the direction of organic chemistry, etc., can solve problems such as not being suitable for industrial production, affecting product yield, and difficulty in product separation, achieving high total yield and purity, improving total yield, The effect of simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of compound Ⅲ
[0038] In the three-necked flask, add 15.72g (0.1mol) of compound II, 300ml toluene and 3.11g (0.1mol) methylamine respectively, stir and raise the temperature to reflux, control the reaction for 1.5h, monitor and track the reaction process by TLC, when the raw materials are completely reacted, Turn off heat. The solvent was removed under reduced pressure to obtain 16.01 g of compound III, the product yield was 94%, and the HPLC purity was 99.96%.
Embodiment 2
[0040] Preparation of compound Ⅲ
[0041] In a three-necked flask, add 15.72g (0.1mol) of compound II, 300ml of dichloromethane and 3.11g (0.1mol) of methylamine, stir and raise the temperature to reflux, and control the reaction for 1.5h. TLC monitors and tracks the reaction progress. After that, stop heating. The solvent was removed under reduced pressure to obtain 15.33 g of compound III, the product yield was 90%, and the HPLC purity was 99.91%.
Embodiment 3
[0043] Preparation of compound Ⅲ
[0044] In the three-necked flask, add compound II 15.72g (0.1mol), dichloromethane 300ml and methylamine 3.42g (0.11mol), stir and heat up to reflux, control the reaction for 1.5h, TLC monitors and tracks the reaction progress, when the raw materials react completely After that, stop heating. The solvent was removed under reduced pressure to obtain 16.34 g of compound III, the product yield was 96%, and the HPLC purity was 99.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com