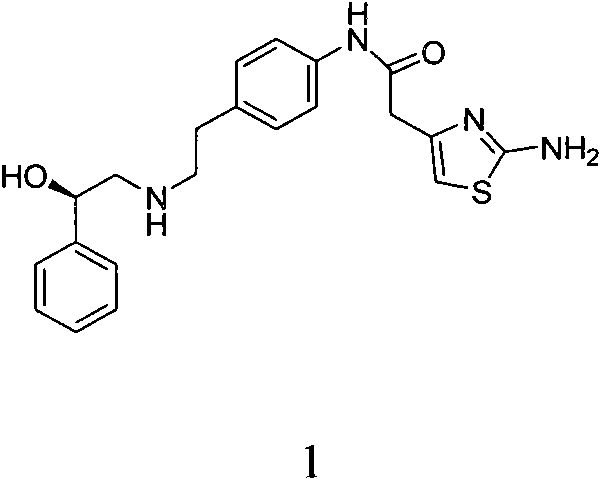
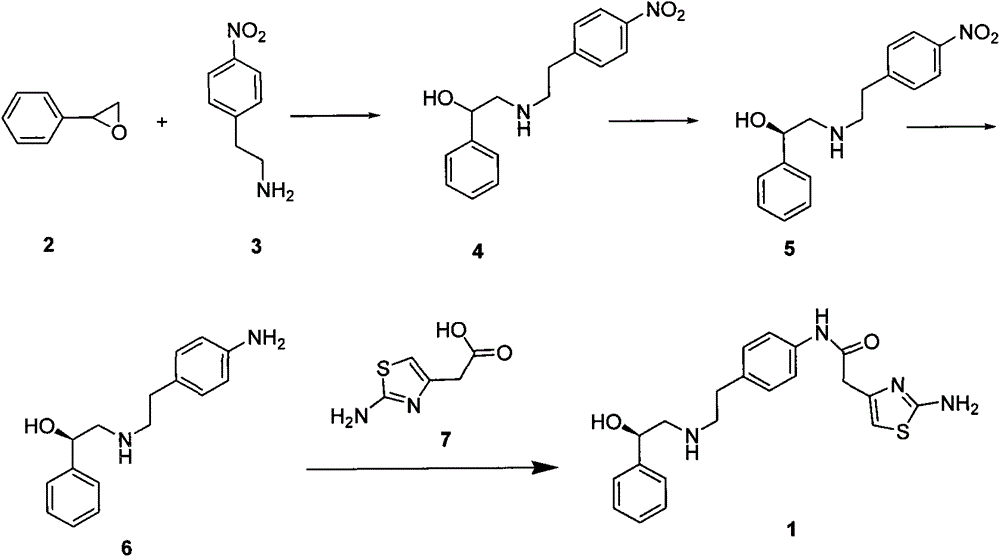
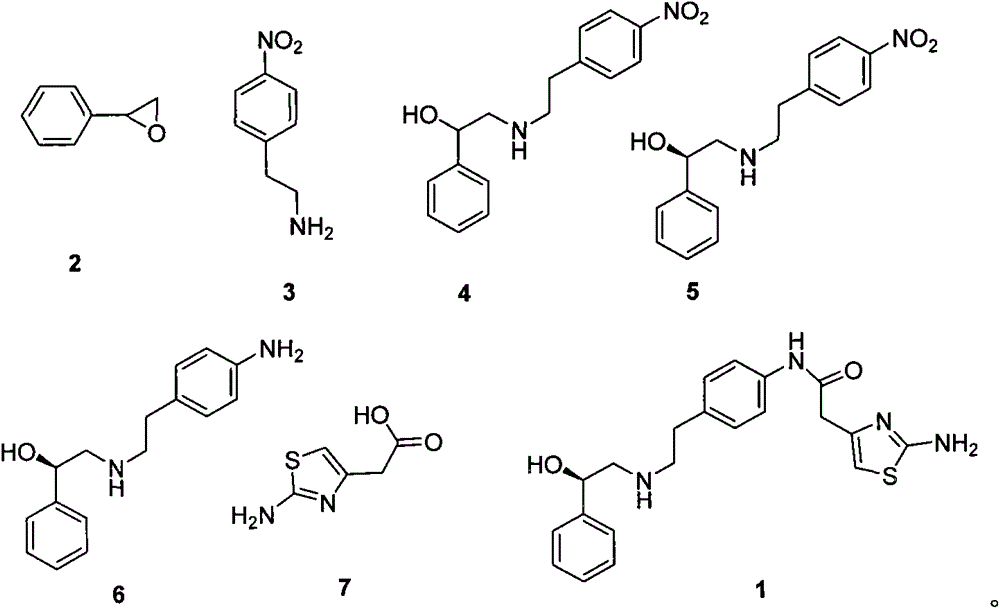
A preparing process of a compound
A compound and process technology, applied in the field of preparing mirabegron, can solve the problems of difficult purification of condensation products, unfavorable industrial production, difficult raw material sources, etc., and achieves simple and easy-to-obtain splitting reagents, short synthetic process routes, and avoidance of high toxicity. effect of chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] At room temperature, dissolve epoxystyrene (12g, 0.1mol) and potassium carbonate (20.7g, 0.15mol) in 1,4-dioxane 100ml, then add p-nitrophenylethylamine (16.6g, 0.1mol ), after the addition, heated to 60°C, reacted for 12 hours, filtered, concentrated to remove most of the dioxane, slowly added to 500ml of water, crystallized, filtered, and dried to obtain 24.20g of white solid, which is formula 4 The compound shown has a yield of 84.73%.
[0037] The analysis data is:
[0038] 1 H-NMR (CDCl 3 , 400MHz): δ8.10-8.20(m, 2H), δ7.41-7.24(m, 7H), δ4.70(d, 1H), δ2.80-3.10(m, 5H), δ2.74( d, 1H).
Embodiment 2
[0040] At room temperature, styrene oxide (12g, 0.1mol) and sodium carbonate (15.9g, 0.15mol) were dissolved in 100ml of acetonitrile, then p-nitrophenylethylamine (15.8g, 0.095mol) was added, after the addition was complete , heated to 55°C, reacted for 10 hours, filtered, concentrated to remove most of the acetonitrile, slowly added to 500ml of water, crystallized, filtered, and dried to obtain 21.87g of white solid, which is the compound shown in formula 4, with a yield of 76.58% .
Embodiment 3
[0042] At room temperature, styrene oxide (12g, 0.1mol) and sodium bicarbonate (12.6g, 0.15mol) were dissolved in 100ml of acetone, then p-nitrophenylethylamine (18.26g, 0.11mol) was added, and the addition was complete Afterwards, heated to 65°C, reacted for 13 hours, filtered, concentrated to remove most of the acetone, slowly added to 500ml of water, crystallized, filtered, and dried to obtain 22.49g of white solid, which was the compound shown in Formula 4, with a yield of 78.75 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com