Synthesis method of 1, 2-dione derivatives
A synthesis method and a technology of derivatives, which are applied in the fields of oxidative preparation of carbonyl compounds, preparation of sulfonic acid amides, organic chemistry, etc., to achieve the effects of high yield, easy availability of raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
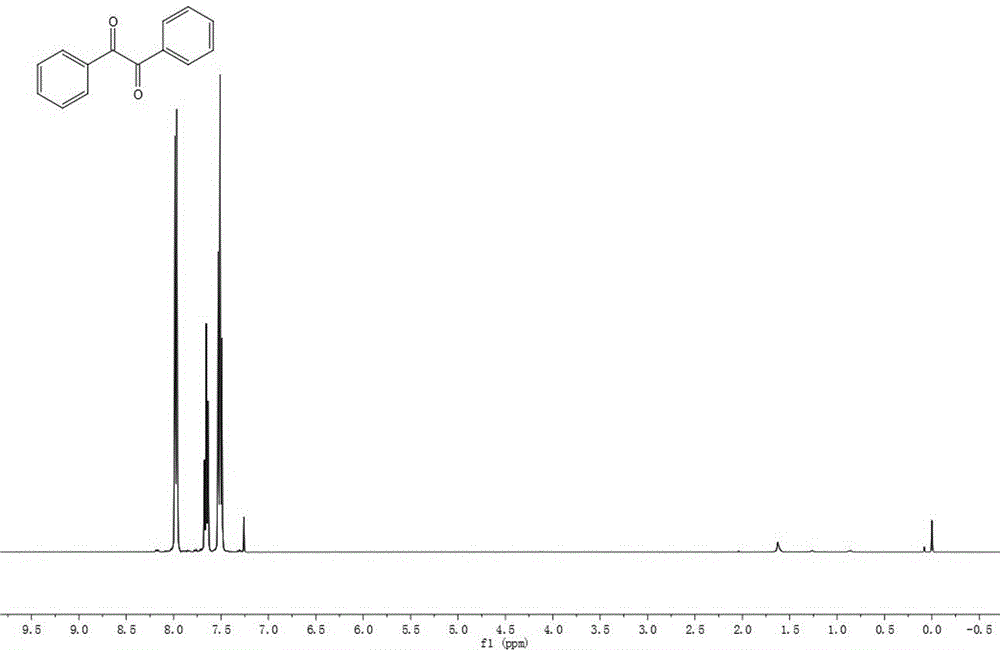
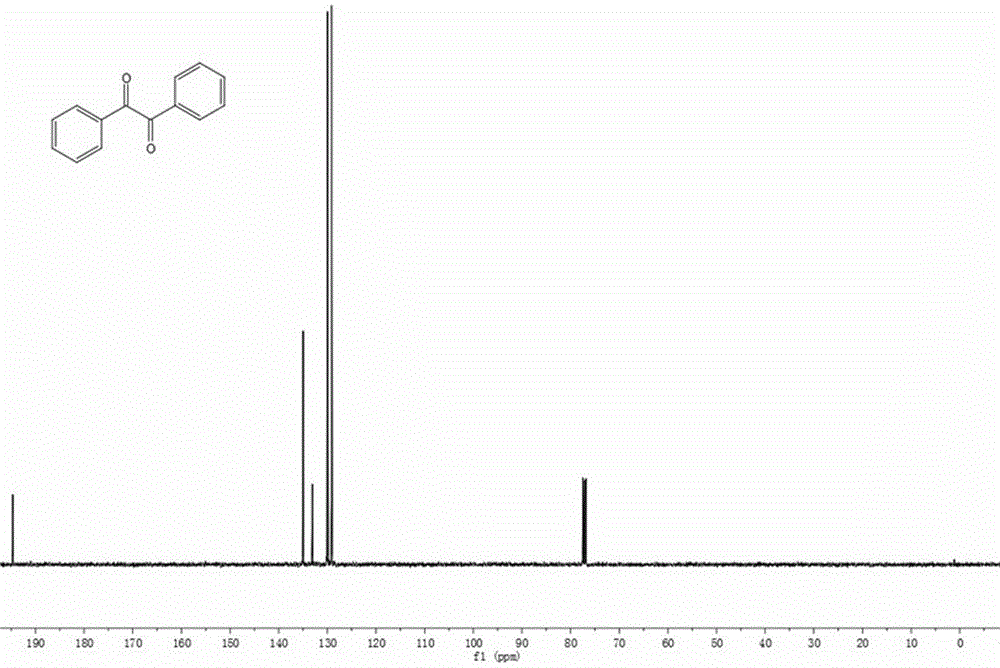
[0024] The synthesis of diphenyl ketone, its structural formula is:
[0025]
[0026] Preparation method 1: In a reaction tube, 12.8 mg of zinc iodide, 35.6 mg of toluene, 71.4 mg of sodium persulfate and 2.0 mL of dimethyl sulfoxide were added under nitrogen protection. Place it in an oil bath at 120°C for 24 hours. Conventional treatment yielded 32.4 mg of pure product with a yield of 77%.
[0027] Preparation method 2: In a reaction tube, add 12.8 mg of zinc iodide, 35.6 mg of toluene, 71.4 mg of sodium persulfate and 2.0 mL of dimethyl sulfoxide under nitrogen protection. Place it in an oil bath at 110°C for 24 hours. Conventional treatment yielded 22.3 mg of pure product with a yield of 53%.
[0028] Preparation method 3: In a reaction tube, add 12.8 mg of zinc iodide, 35.6 mg of toluene, 71.4 mg of sodium persulfate and 2.0 mL of dimethyl sulfoxide in an air atmosphere. Place it in an oil bath at 120°C for 24 hours. Conventional treatment yielded 31.5 mg of pure ...
Embodiment 2
[0035] Synthesis of 1-phenyl-2-(4-methylphenyl)-1,2,-dione, whose structural formula is:
[0036]
[0037] In a reaction tube, 12.8 mg of zinc iodide, 38.5 mg of 1-phenyl-2-(4-methylphenyl)acetylene, 71.4 mg of sodium persulfate and 2.0 mL of dimethyl sulfoxide were added under nitrogen protection. Place it in an oil bath at 120°C for 24 hours. Conventional treatment yielded 27.4 mg of pure product with a yield of 61%.
[0038] The result of NMR characterization of the product is: 1 H NMR (400MHz, CDCl 3 )δ7.97(dd, J=10.8,3.5Hz,2H),7.87(d,J=8.1Hz,2H),7.65(t,J=7.4Hz,1H),7.50(t,J=7.7Hz, 2H), 7.31(d, J=8.0Hz, 2H), 2.43(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ 22.04, 129.10, 129.14, 129.86, 130.00, 130.14, 130.71, 133.22, 134.90, 146.34, 194.42, 194.89.
Embodiment 3
[0040] Synthesis of 1-(4-methoxyphenyl)-2-phenyl-1,2,-dione, whose structural formula is:
[0041]
[0042] In the reaction tube, 12.8 mg of zinc iodide, 41.7 mg of 1-(4-methoxyphenyl)-2-phenylacetylene, 71.4 mg of sodium persulfate and 2.0 mL of dimethyl sulfoxide were added under nitrogen protection. Place it in an oil bath at 120°C for 24 hours. Conventional treatment yielded 41.8 mg of pure product with a yield of 87%.
[0043] The result of NMR characterization of the product is: 1 H NMR (400MHz, CDCl 3 )δ8.00-7.92 (m, 4H), 7.64 (t, J = 7.4Hz, 1H), 7.50 (t, J = 7.9Hz, 2H), 6.97 (d, J = 9.0Hz, 2H), 3.88 ( s,3H); 13 C NMR (100MHz, CDCl 3 )δ55.76, 114.49, 126.20, 129.06, 129.99, 132.48, 133.31, 134.82, 165.11, 193.28, 194.97.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com