Patents
Literature
50 results about "Zinc iodide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zinc iodide is a chemical compound of zinc and iodine, ZnI₂. The anhydrous form is white and readily absorbs water from the atmosphere. It can be prepared by the direct reaction of zinc and iodine in refluxing ether.
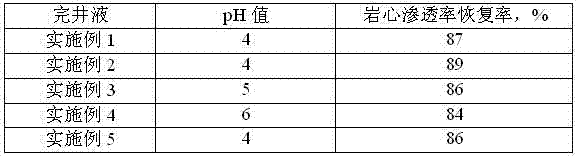
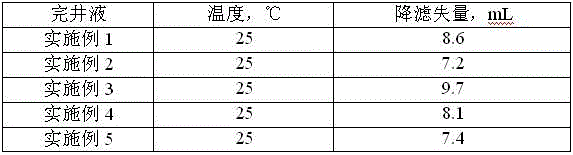
Novel high density brines for completion applications
Clear, high density brine for use completion operations in a subterranean formation for the recovery of hydrocarbons. The brine comprises an ionic compound selected from the group consisting of zinc iodide, strontium bromide, strontium iodide, cerium bromide, cerium iodide, cerium chloride, lanthanum bromide, lanthanum iodide, lanthanum chloride, and mixtures thereof. The brine may also advantageously be used as the internal phase of invert emulsion drilling fluids.
Owner:HALLIBURTON ENERGY SERVICES INC
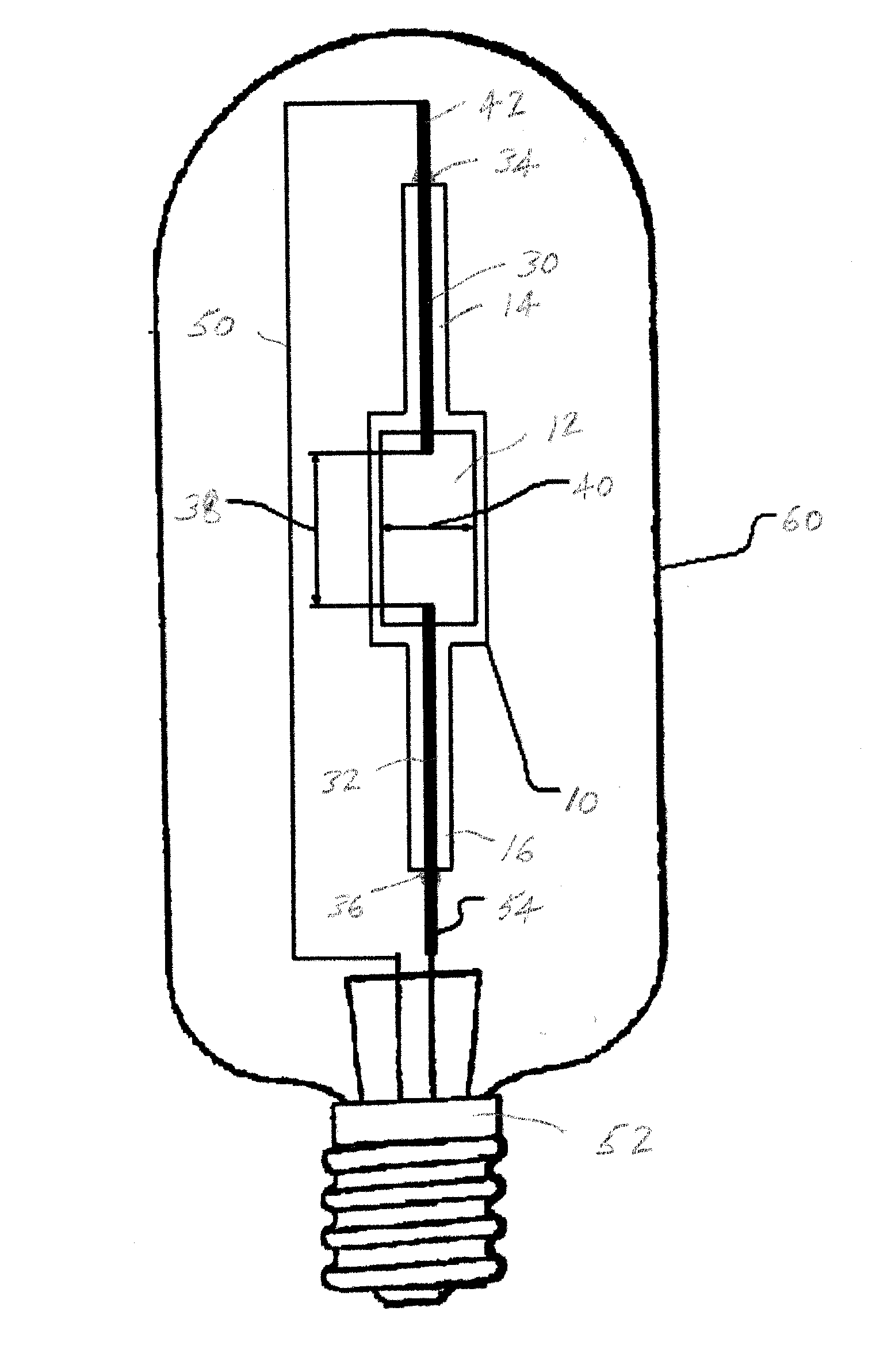
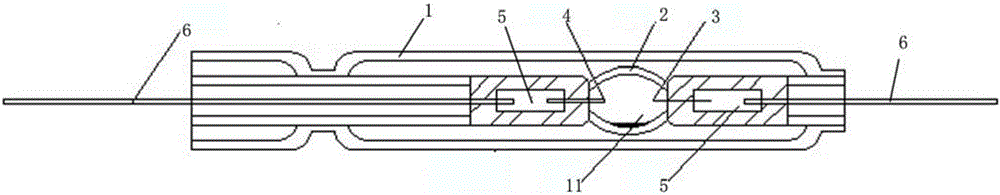
Mercury free discharge lamp with zinc iodide
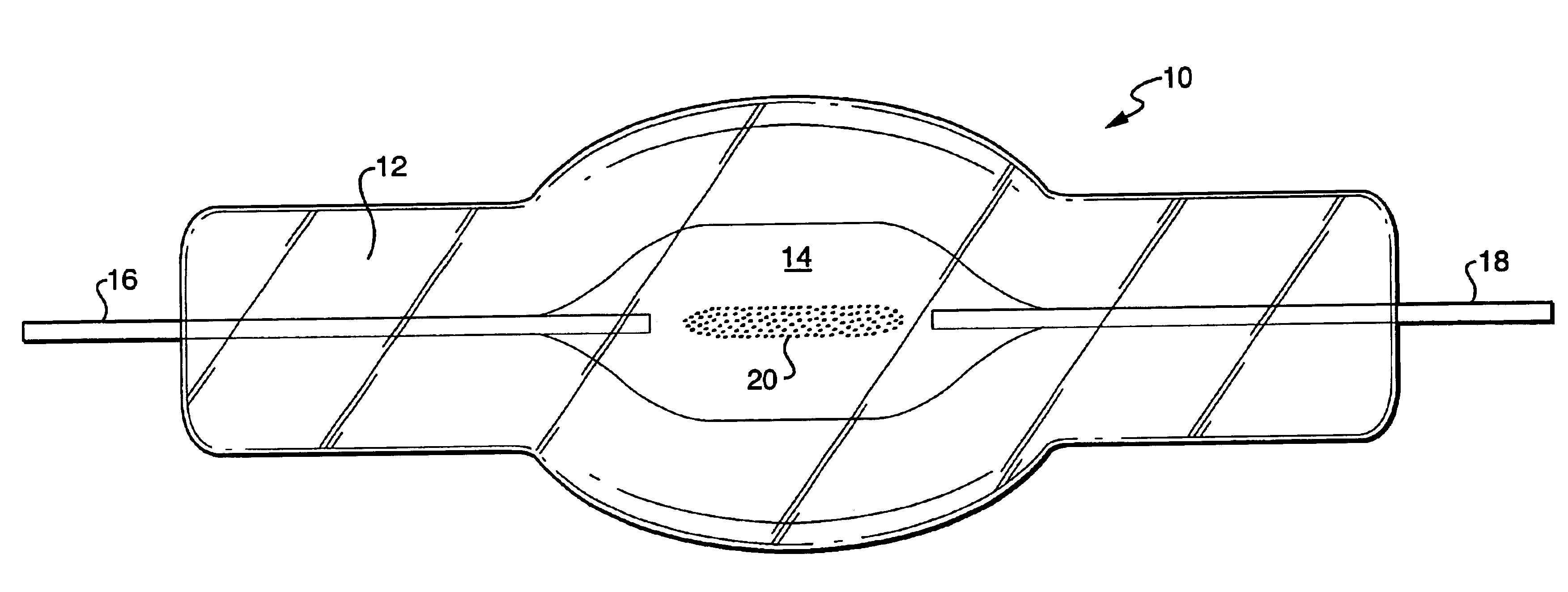
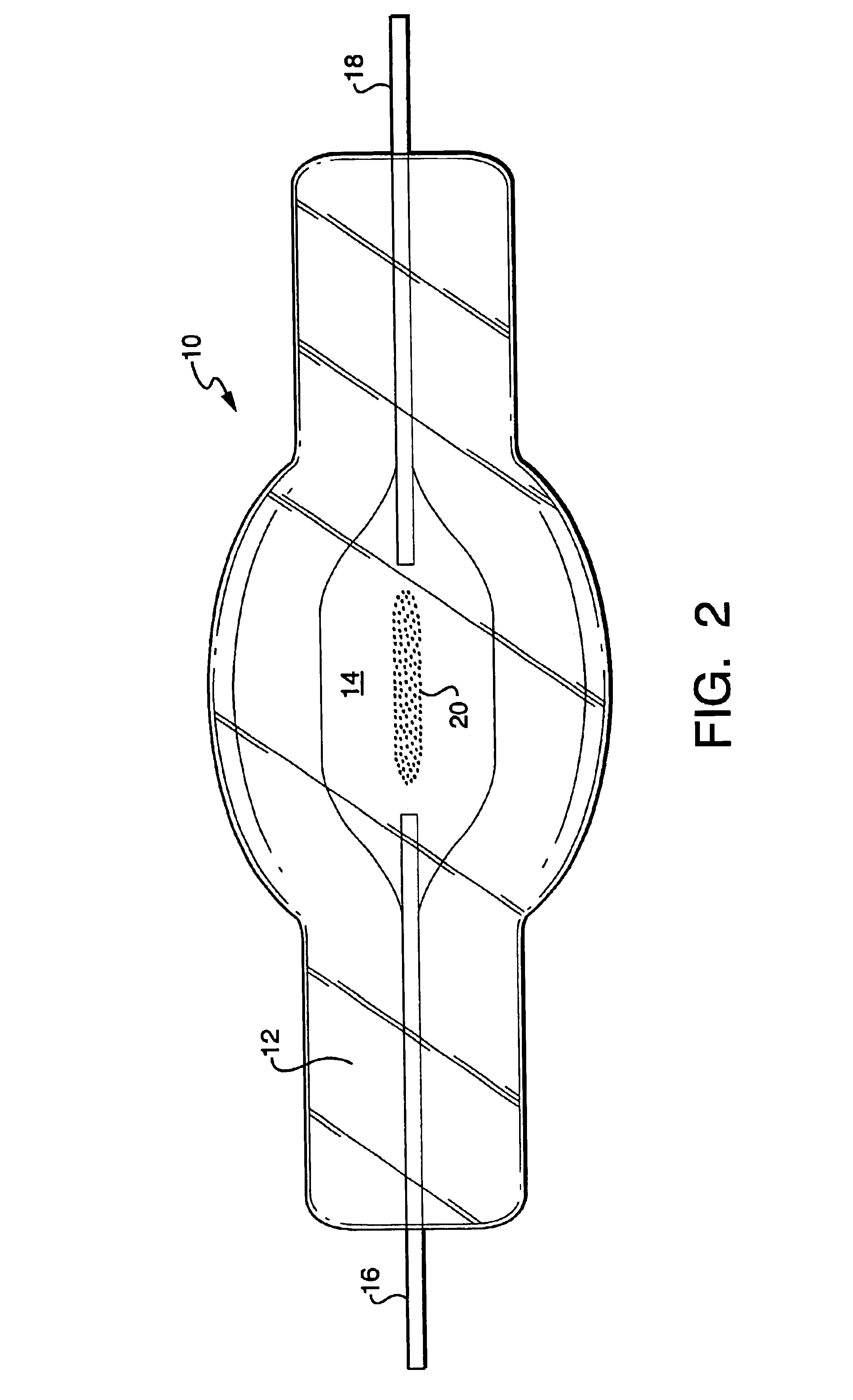
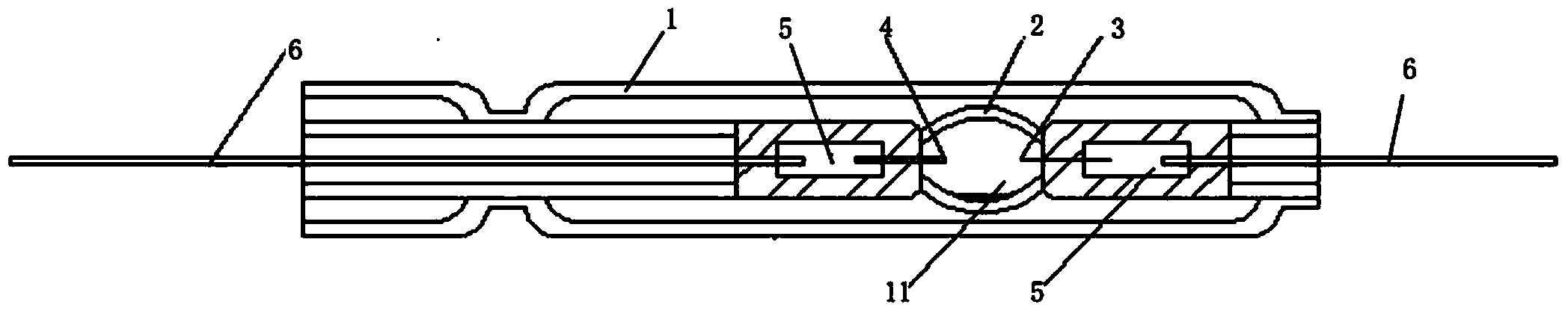
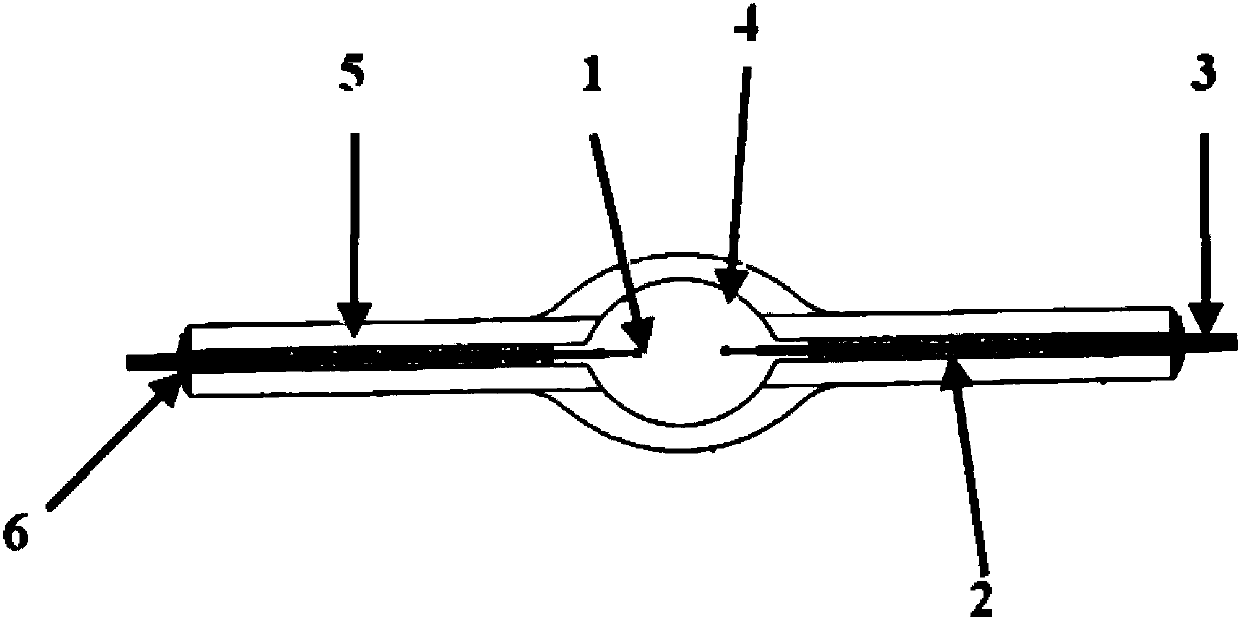
A compact fused silica, electroded HID lamp for automotive forward lighting, which contains no mercury. The lamps voltage, approximately 40 volts, is developed in this lamp by vaporizing zinc iodide instead of mercury. A compromise between voltage and luminous flux is achieved through the choice of the sodium scandium (Na:Sc) molar ratio, between 4.5:1 and 6:1 and a zinc iodide (ZnI2) dose of 2 to 6 micrograms per cubic millimeter that permits the lamp to operate within the North America, European and Japanese automotive color specifications for white light. The voltage in the lamp can be controlled according to the zinc iodide doping level without seriously impacting the visible spectrum otherwise provided by the other known dopants in the lamp.
Owner:OSRAM SYLVANIA INC
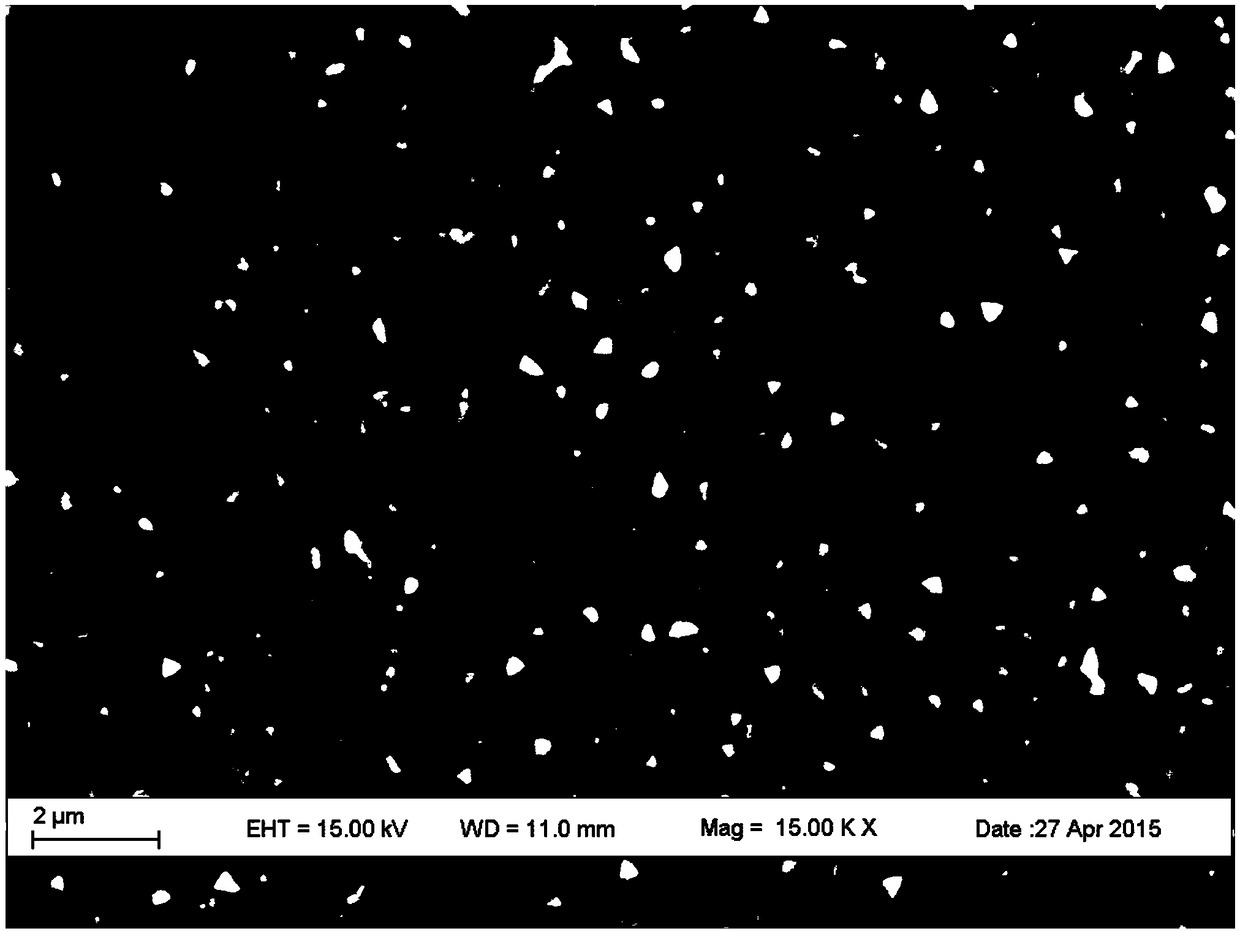
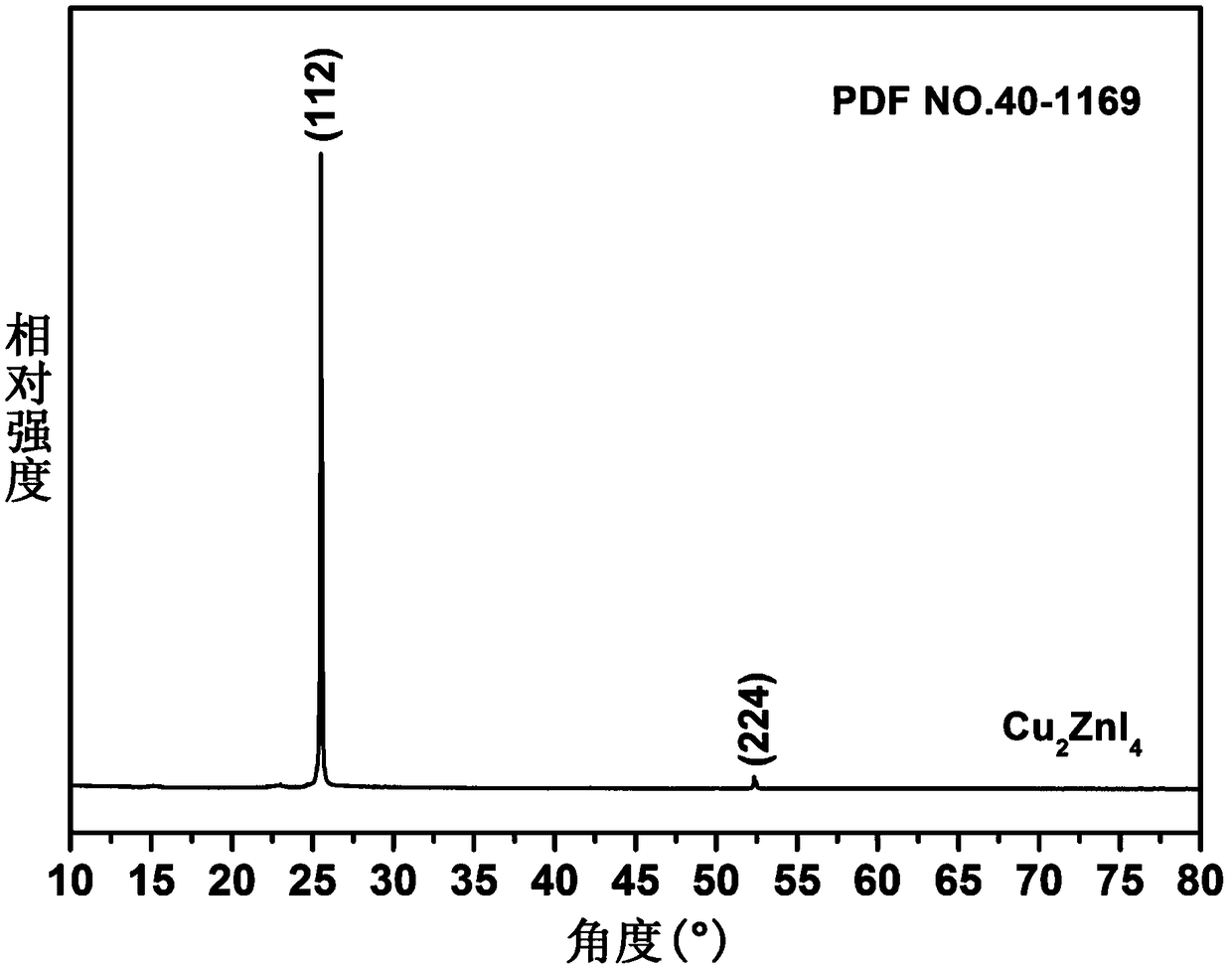
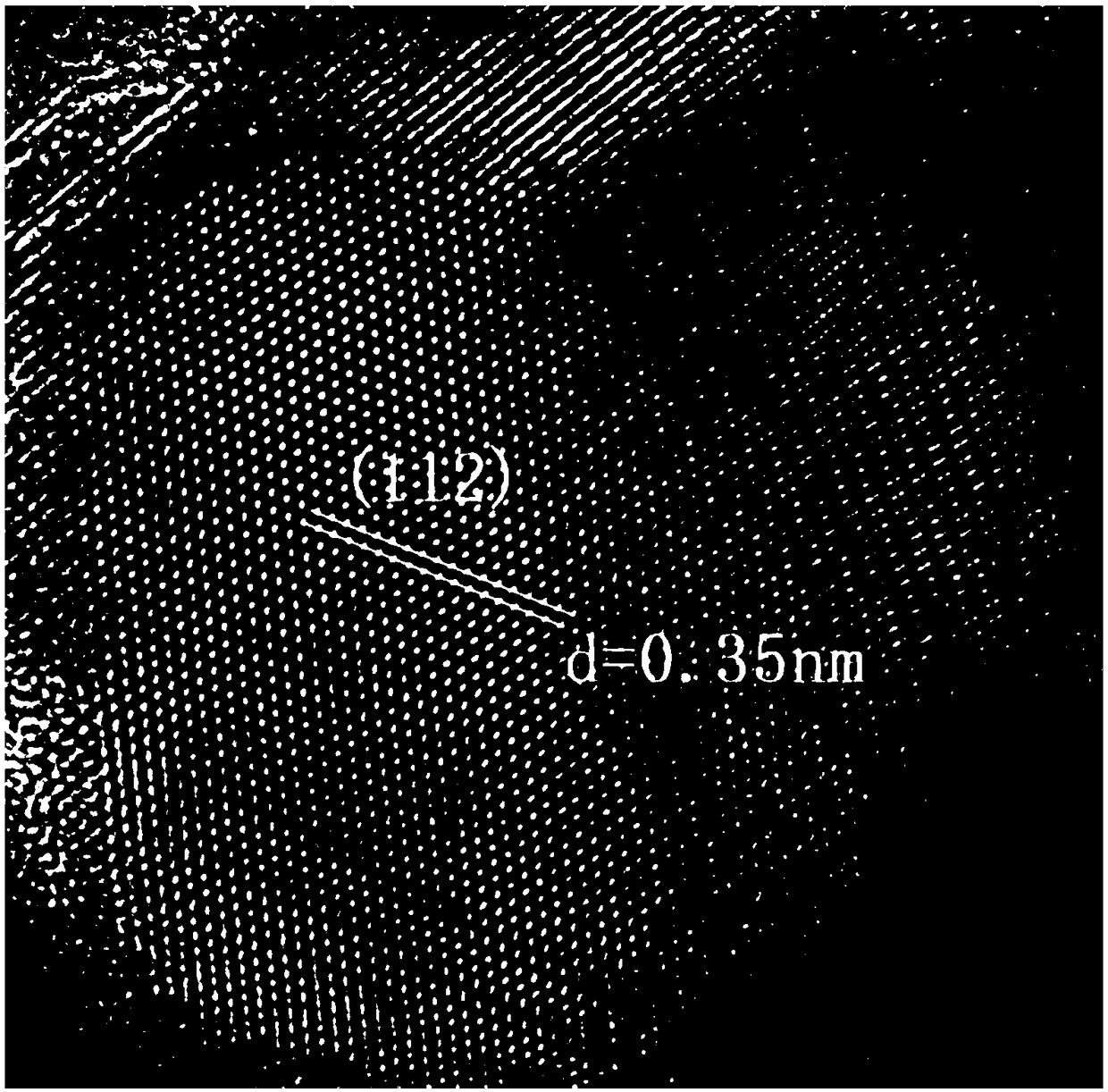
Chemical method for semiconductor film materials of ternary wide bandgap compound of synthesis of copper-zinc iodide
ActiveCN106449367ALow costGood reproducibilitySemiconductor/solid-state device manufacturingZinc alloysFilm material
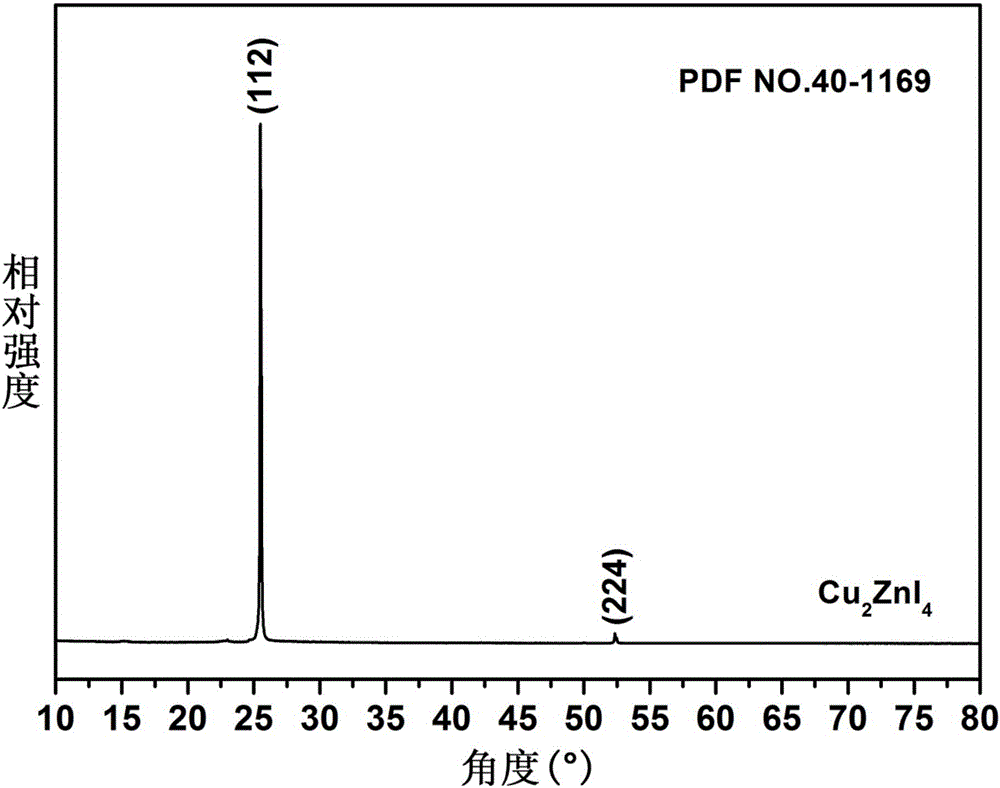
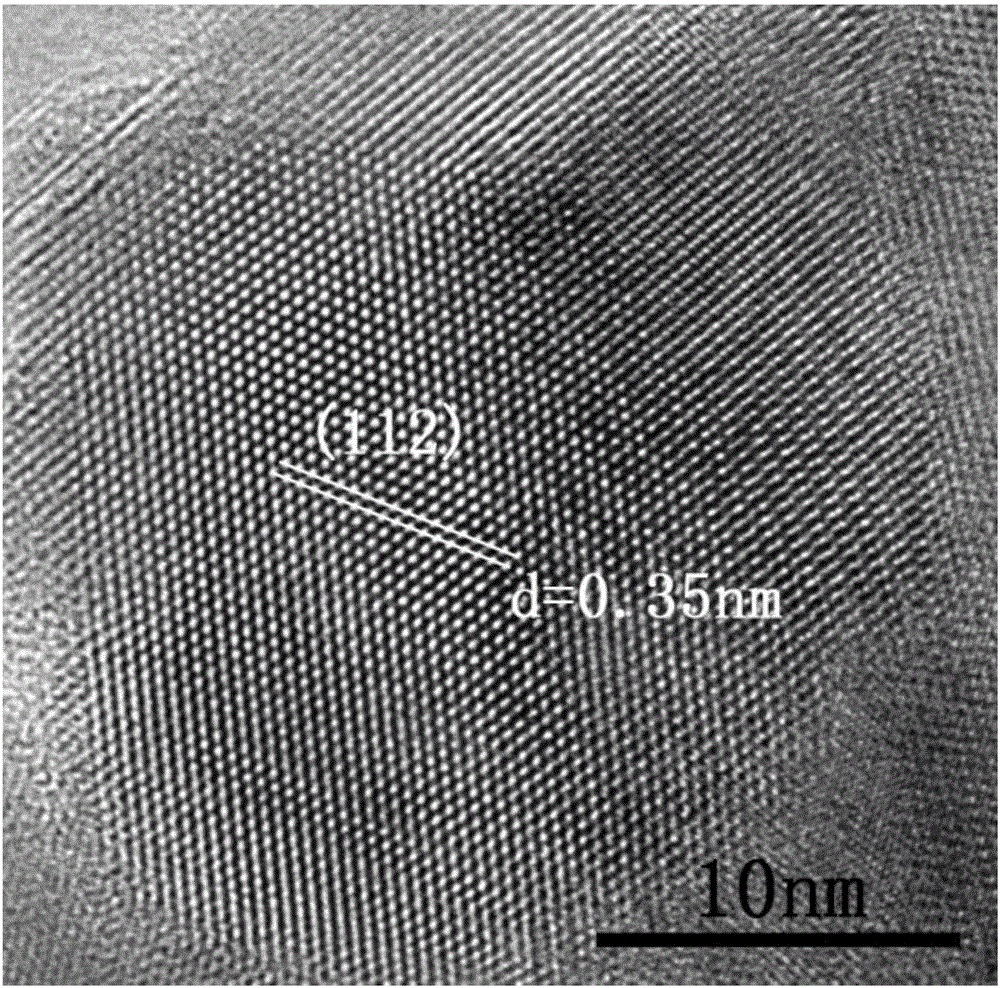
The invention belongs to chemical technical field, in particular to a chemical method for semiconductor film materials of ternary wide bandgap compound of synthesis of copper-zinc iodide. The method is that putting the copper-zinc alloy film into iodine vapor with a temperature of 45 DEG C to 80 DEG C for co-oxidation reaction of the copper-zinc alloy. The reaction time takes 3 to 8 hours. After reacting for certain time, Cu2ZnI4 films can be produced in situ on the surface of substrate, and that is the semiconductor film materials of the synthesis of the copper-zinc iodide. The preparation method does not require organic solvents to participate in the reaction or reaction medium. The crystal is crystallized well. The product can be used directly without complicated processing. The method has the advantages of simple operation, rapid reaction, better environmental protection, low energy consumption, low cost and good reproducibility. In addition, a film can be directly formed on the substrate surface in the method. The material is more beneficial for the application in photoelectric conversion devices.
Owner:XUCHANG UNIV
Low-permeability solid-free completion fluid
Owner:DAQING HUAYING CHEM IND
Preparation of goldless green phosphor
InactiveCN101376807AIncrease brightnessHigh chromaLuminescent compositionsStrontium bromideFluorescence
The invention relates to a manufacture method of barren green fluorescent powder. In the method, a latent solvent system consisting of barium iodide, zinc iodide, potassium iodide and strontium bromide is adopted; activated aluminum Gamma-Al2O3 is used as the leading-in matter of a coactivator Al; copper nitrate is used as the leading-in matter of an activator Cu; the led-in Cu is used to replace the heavy metal gold which belongs to the same main group of the Cu, thus realizing to manufacture the barren green powder the chroma X value of which is between 0.293 and 0.305 and extensively reducing the manufacture cost of the fluorescent powder; besides, the brightness of the fluorescent powder is improved by 2 percent; the color of the fluorescent powder is purer and plumper.
Owner:CAIHONG GRP ELECTRONICS CO LTD
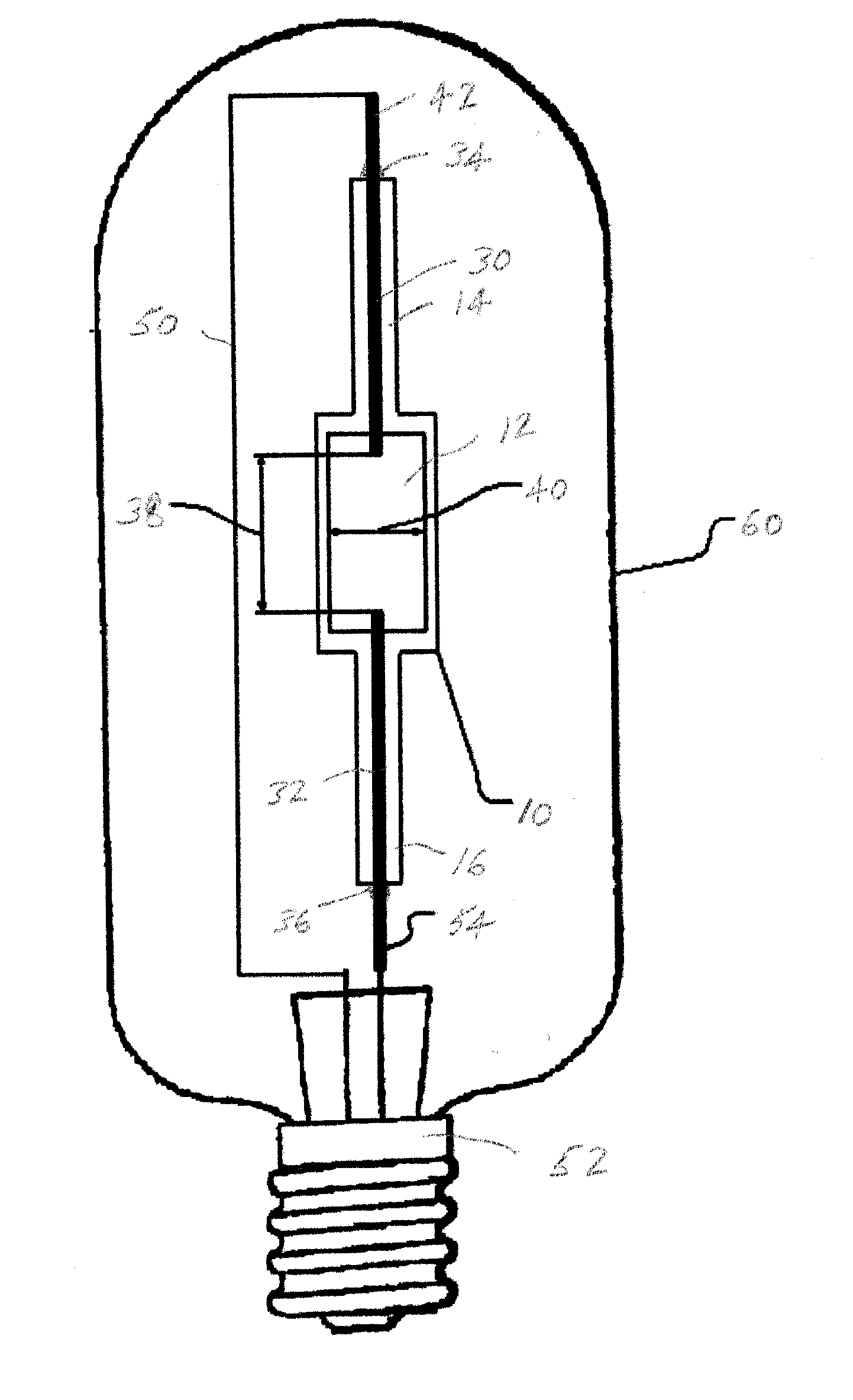
Mercury-free metal halide discharge lamp
InactiveUS20080018254A1Reduce the amount requiredLow total lumen outputSolid cathode detailsGas discharge lamp detailsNoble gasElectric light
A metal halide discharge lamp comprises a lamp body and a chamber formed within the body. A pair of electrodes extends into the chamber and have electrode tips spaced apart from one another. A discharge medium composition is sealed within the chamber that generates a plasma, which generates visible light. The composition comprises a rare gas, a first metal halide that produces a luminous flux and zinc iodide that generates a desired lamp operating voltage. The composition may also comprise zinc, sealed in the chamber, in elemental form that is not derived from the first metal halide or the zinc iodide. The zinc iodide halide serves as a substitute for mercury for purposes of generating desired lamp operating voltage; and, the excess pure zinc attracts or reacts with iodine atoms thereby making available electrons and the first metal halide for generation of a luminous flux.
Owner:GENERAL ELECTRIC CO
Small-power metal halide direct current lamp
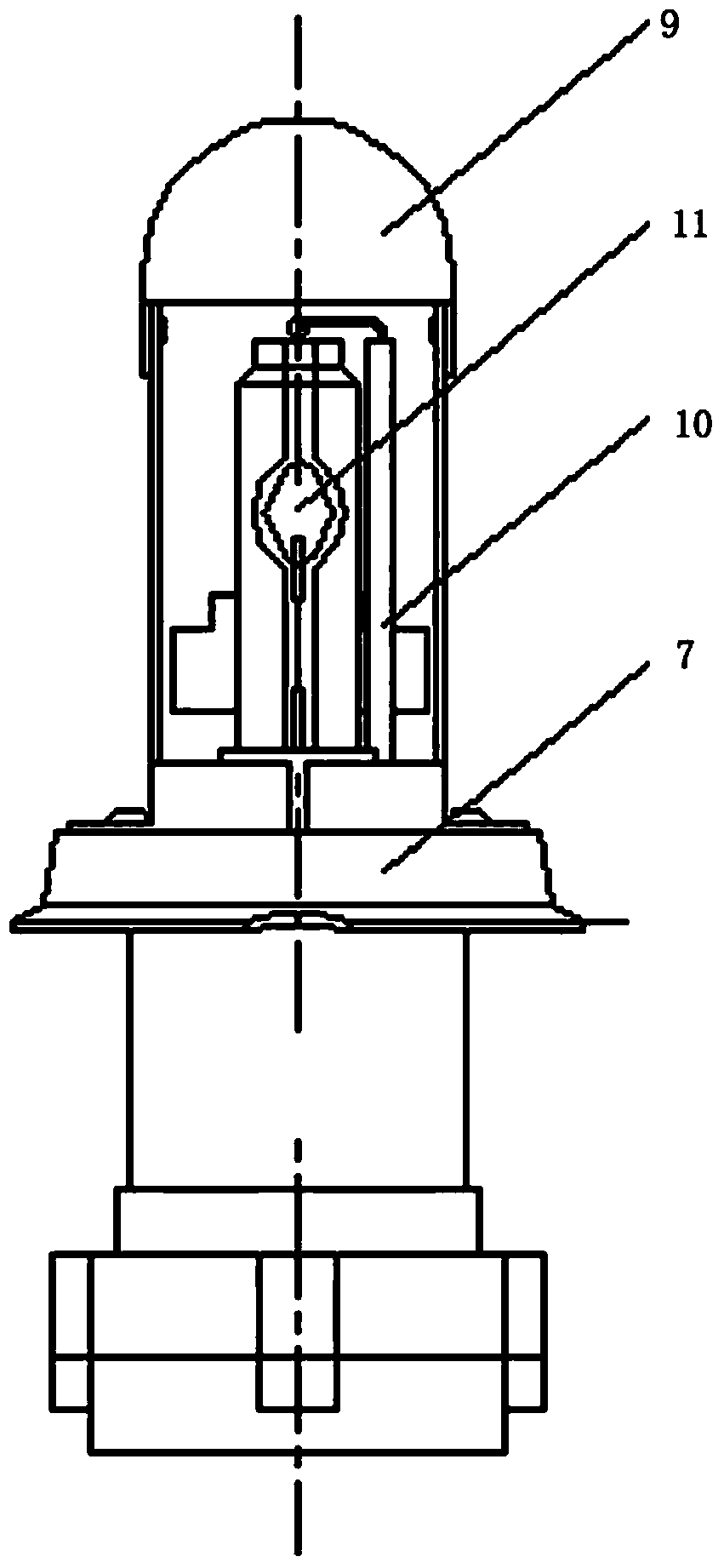
ActiveCN103456598AStable light outputStable discharge arcGas discharge lamp detailsMetal halidesZinc iodide
The invention belongs to the technical field of illumination of light sources, and particularly relates to a small-power metal halide direct current lamp which is stable in arc and can work under a direct current stabilizer. The small-power metal halide direct current lamp comprises a quartz bubble shell, wherein a sealed light-emitting arc tube is arranged in the quartz bubble shell, a cathode and an anode are arranged in the sealed light-emitting arc tube, one end of the cathode extends into the light-emitting arc tube, the anode is matched with the cathode, the other end of the cathode and the other end of the anode are located in the quartz bubble shell and are connected with molybdenum sheets respectively, the molybdenum sheets are connected with molybdenum rod leads respectively, the molybdenum rod leads extend out of the quartz bubble shell, the light-emitting arc tube is filled with stuffing, the stuffing comprises 3-6.5 atmospheres of xenon, 0.5-0.9mg of mercury, zinc iodide and one or more of metal halide DyI3, DyBr3, HoBr3, NdI3, CsI, InI, NaI, ScI3, ThI4 and T1I. The small-power metal halide direct current lamp can output stable light rays, bring convenience to using and reduce using cost of users, and is long in service life.
Owner:CHANGZHOU NEWPHENIX LIGHTING MFR
Preparation method of thioester compounds
ActiveCN112239384AEasy to prepareSimple stepsFunctional group formation/introductionSulfonyl chlorideTriflic acid
The invention discloses a preparation method of thioester compounds, wherein the method comprises the following steps: adding nickel trifluoromethanesulfonate, 4,4'-di-tert-butyl-2,2'-dipyridyl, carbonyl molybdenum, potassium carbonate, zinc iodide, water, arylboronic acid and sulfonyl chloride into an organic solvent, gathering, reacting at the temperature of 120 DEG C for 16 hours, and after thereaction is completed, carrying out after-treatment to obtain the thioester compound. The preparation method has the advantages of simple operation, cheap and easily available reaction starting raw materials, strong substrate designability, wide substrate functional group tolerance range, high reaction efficiency, and no need of additional oxidants and reducing agents by using sulfonyl chloride as a sulfur source. Various thioester compounds can be synthesized according to actual requirements, so that the practicability of the method is widened while the operation is convenient.
Owner:ZHEJIANG SCI-TECH UNIV
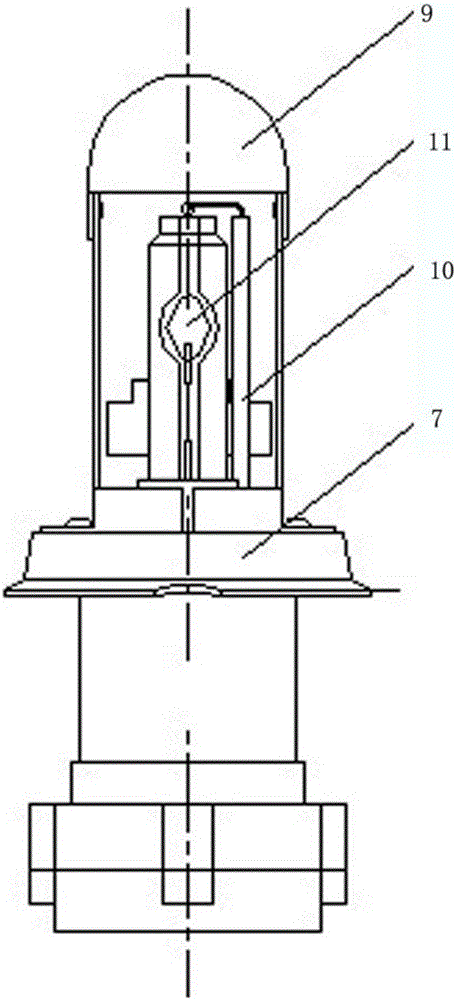
Low mercury ceramic metal halide lamp
This disclosure relates to a low mercury content, metal halide lamp having a ceramic arc tube with an aspect ratio ranging from approximately 1.15 up to about 4.75, and having a fill in the arc tube of up to 2 milligrams of mercury per cubic centimeter and up to 10 milligrams of zinc metal and / or zinc / zinc iodide per cubic centimeter.
Owner:GENERAL ELECTRIC CO
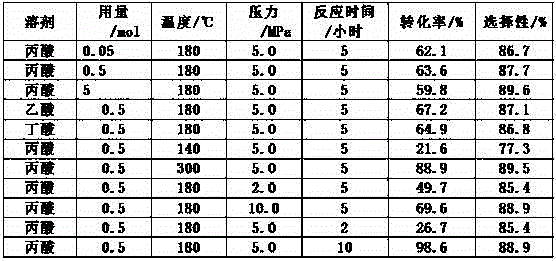
Method for preparing propionic acid from ethyl acetate through carbonylation
InactiveCN102911035AOvercome the priceOvercome the disadvantages of increasingCarboxylic preparation from carbon monoxide reactionPropanoic acidIodide
The invention discloses a method for preparing propionic acid from ethyl acetate through carbonylation. A reaction system consists of ethyl acetate, a main catalyst, a co-catalyst and water according to a molar ratio of 1:0.01-0.0001:0.05-0.5:1-10, wherein the reaction temperature is 140-300 DEG C, and the carbon monoxide pressure is 2.0-10.0 MPa; the main catalyst is a rhodium compound; the co-catalyst is iodoethane; an iodide additive such as hydriodic acid, lithium iodide, potassium iodide, tin iodide, lead iodide, zinc iodide or cadmium iodide, a polar solvent such as acetic acid, propionic acid or butyric acid and an organic ligand are selectively added into the reaction system; the molar ratio of the iodide additive to the ethyl acetate is 0.01-1; the molar ratio of the polar solvent to the ethyl acetate is 0.1-10; and the molar ratio of the organic ligand to the main catalyst is 1-5. The process raw materials are freely and economically selected, the problem that ethanol is hydrolyzed and esterified to produce diethyl ether and propionic acid under an acidic condition is solved, and the selectivity of the propionic acid is improved.
Owner:JIANGSU SOPO GRP +1
Method for continuous conversion of methanol to higher hydrocarbons and catalyst used therein
InactiveUS20110046426A1Inexpensive and continuous and efficientImprove efficiencyCatalystsHydrocarbon preparation catalystsIndiumDistillation
Methods and apparatuses for converting methanol to higher hydrocarbons in a continuous process. A distillation column may be packed with inert material and filled with an ionic liquid. The ionic liquid may function as both reaction medium and catalyst. Derivative of zinc iodide and indium iodide may serve as the possible catalytic species. Higher hydrocarbons may be isolated from reaction effluent by condensation in a cold-water condenser, a cold trap, or both.
Owner:HAMPDEN SYDNEY COLLEGE
OSP copper surface antioxidant and preparation method thereof
InactiveCN109487257ARapid responseImprove shineMetallic material coating processesNon-metallic protective coating applicationAcetic acidAntioxidant
The invention discloses an OSP copper surface antioxidant. The OSP copper surface antioxidant is prepared form the following raw materials in parts by weight: 0.2-5kg of copper acetate, 0.5-5kg of zinc acetate, 1-8kg of dip-chlorobenzyl benzimidazole, 0.5-10kg of heptanoic acid, 20-200kg of glacial acetic acid, 1-20kg of formic acid and 0.001-0.1kg of zinc iodide. A preparation method of the OSP copper surface antioxidant comprises the specific preparation steps that a solution (1) is prepared, specifically, 1.1, a small stirring barrel is taken and added with glacial acetic acid, and the formic acid is added and stirred evenly; 1.2, heptanoic acid is added slowly and stirred, continually stirring is carried out for 30 minutes; 1.3, dip-chlorobenzyl benzimidazole and zinc acetate are addedand fully stirred for 60 minutes until solids are completely dissolved, and thus the solution (1) is obtained; 1.4, a small amount of the solution (1) is utilized to clean a heptanoic acid measuringtool in the solution (1) step; 1.5, the solution (1) is added into a large stirring barrel; a solution (2) is prepared, specifically, 1.6, 850 L of pure water is taken, copper acetate is completely dissolved, stirring is carried out for 60 minutes, and the solution (2) is obtained; and 1.7, the solution (2) is slowly added into the large stirring barrel containing the solution (1), stirring is carried out while adding is carried out, uniform stirring is carried out, and the zinc iodide is slowly added for not less than 20 minutes. The OSP copper surface antioxidant has the advantages of low cost and good high temperature resistance.
Owner:南通赛可特电子有限公司
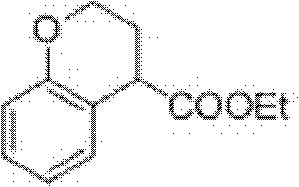
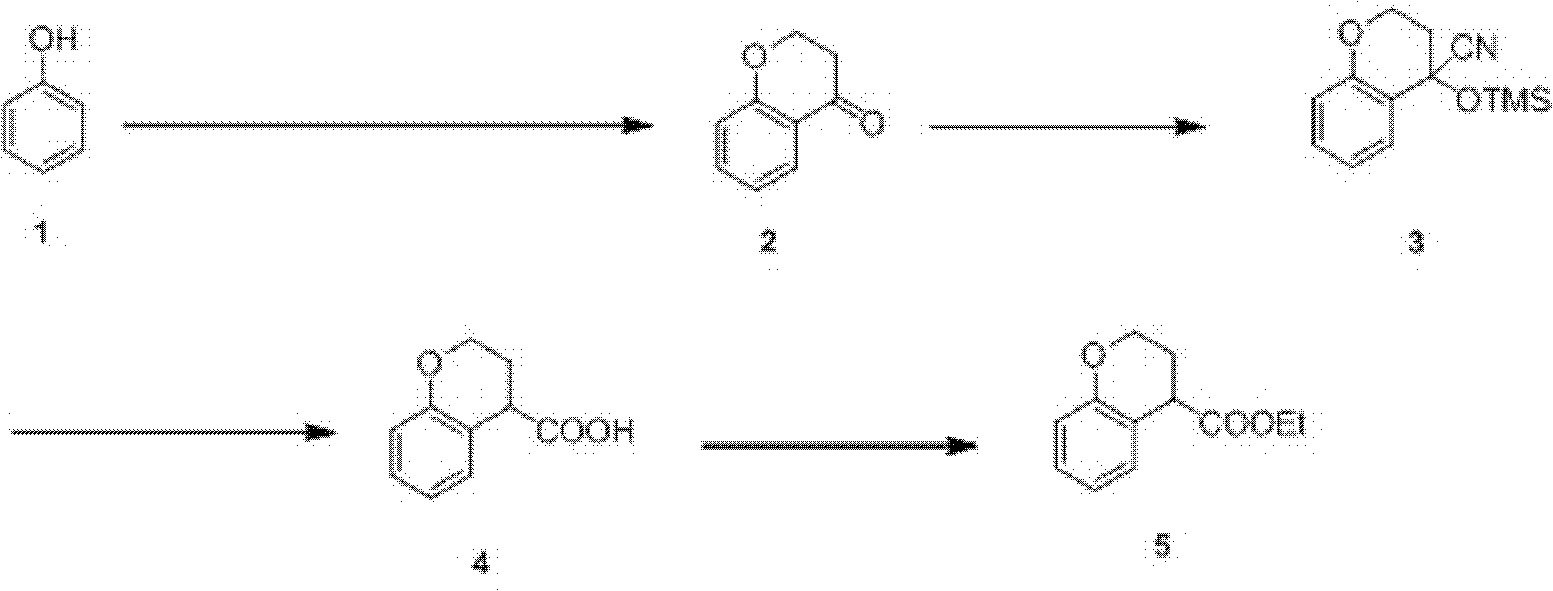
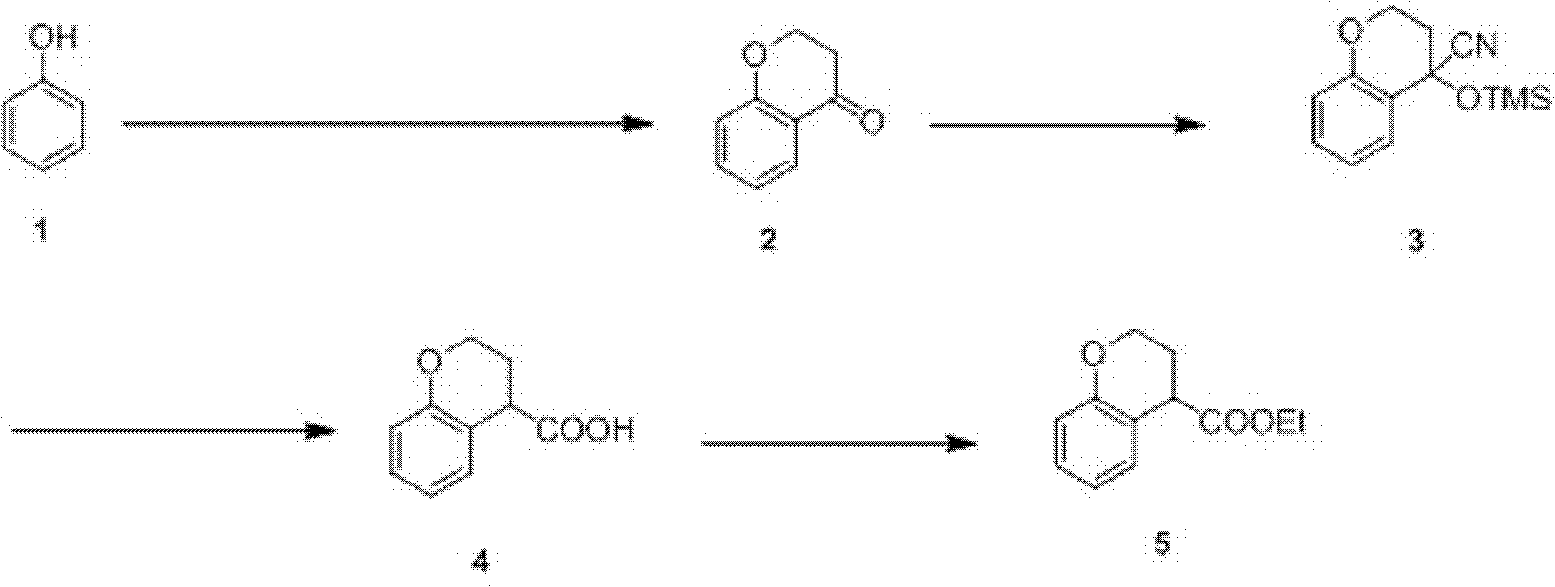
Preparation process of ethyl chromane-4-formate
The invention relates to a preparation process of ethyl chromane-4-formate. The preparation process is characterized by comprising the following steps of: carrying out Friedel-Crafts acylation reaction and cyclization, addition reaction, hydrolysis reaction and esterification reaction, wherein, in the Friedel-Crafts acylation reaction and cyclization, the Friedel-Crafts acylation reaction is carried out in the presence of anhydrous aluminium trichloride by using a compound of formula 1 and 3-chloropropionylchloride as initial raw materials, then carrying out cyclization on an obtained product and 10% NaOH to obtain a compound of formula 2; in the addition reaction, the addition reaction is carried out on the compound of formula 2 and trimethylsilyl cyanide in the presence of zinc iodide to obtain a compound of formula 3; in the hydrolysis reaction, the compound of formula 3 is hydrolyzed under the conditions of stannous chloride dihydrate, concentrated hydrochloric acid and acetic acid to obtain a compound of formula 4; and in the esterification reaction, the compound of formula 4 is esterified in the presence of concentrated sulfuric acid to obtain a compound of formula 5. According to the preparation process, expensive trifluoromethanesulfonic acid is replaced with cheap anhydrous aluminium trichloride in the Friedel-Crafts acylation step, and the yield is improved, so that the cost of preparing the compound is greatly reduced, and the high-quality product is obtained; and compared with the traditional method, the preparation process has obvious advantages.
Owner:苏州莱克施德药业有限公司
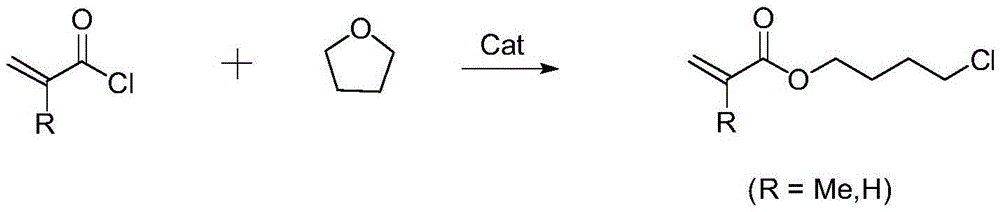
Preparation method of acrylic acid-4-chlorobutyl ester compounds
InactiveCN105646213AHigh yieldPreparation reaction conditions are mildOrganic compound preparationCarboxylic acid esters preparationZinc bromideOrganic solvent
The invention discloses a preparation method of acrylic acid-4-chlorobutyl ester compounds. A catalyst is added to an organic solvent, acryloyl chloride or methacryloyl chloride is added dropwise, the mixture undergoes a ring-opening reaction for 0.5-10 h, and acrylic acid-4-chlorobutyl ester compounds are generated; the catalyst is a zinc salt compound. With the zinc salt compound such as zinc chloride, zinc bromide or zinc iodide as the catalyst, the preparation reaction condition is relatively mild, and the acrylic acid-4-chlorobutyl ester product can be obtained in a short time, and the yield of the acrylic acid-4-chlorobutyl ester product prepared with the method is higher and can be 90% or higher.
Owner:江苏优普生物化学科技股份有限公司
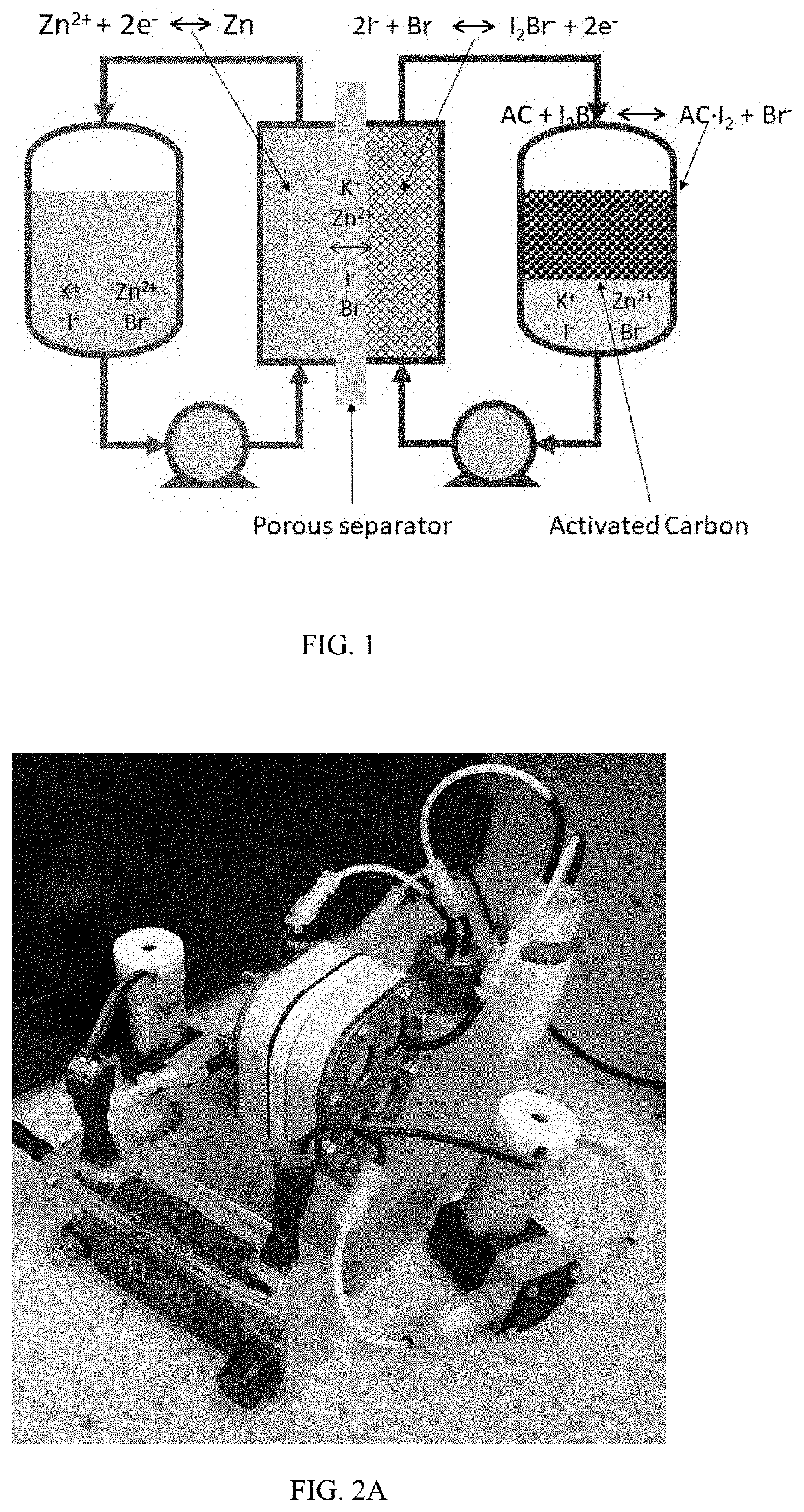
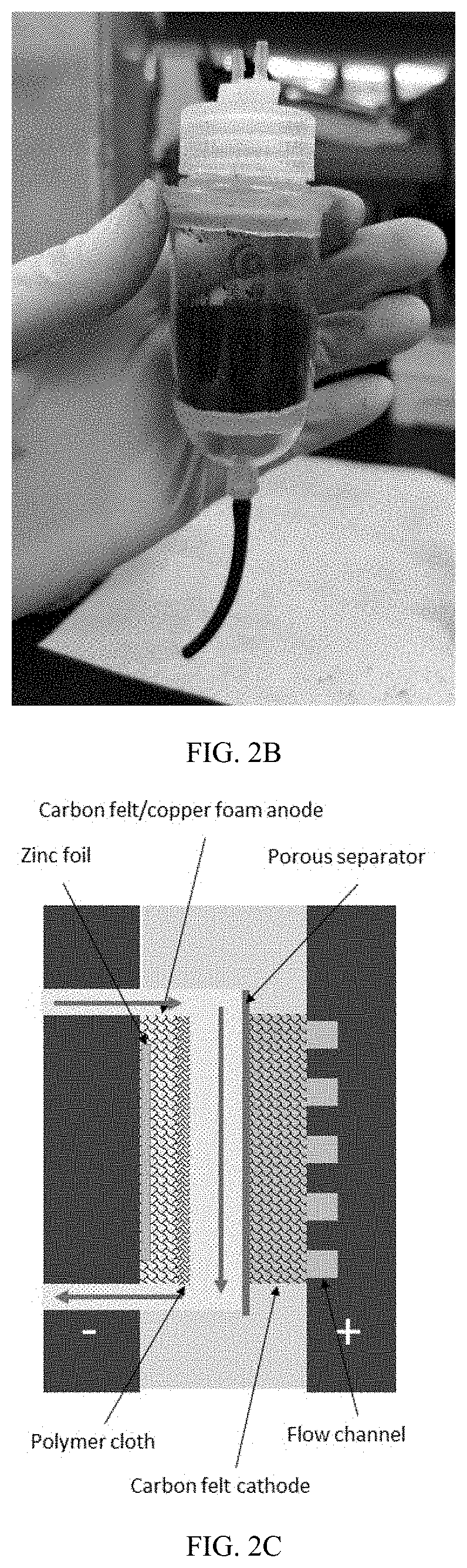
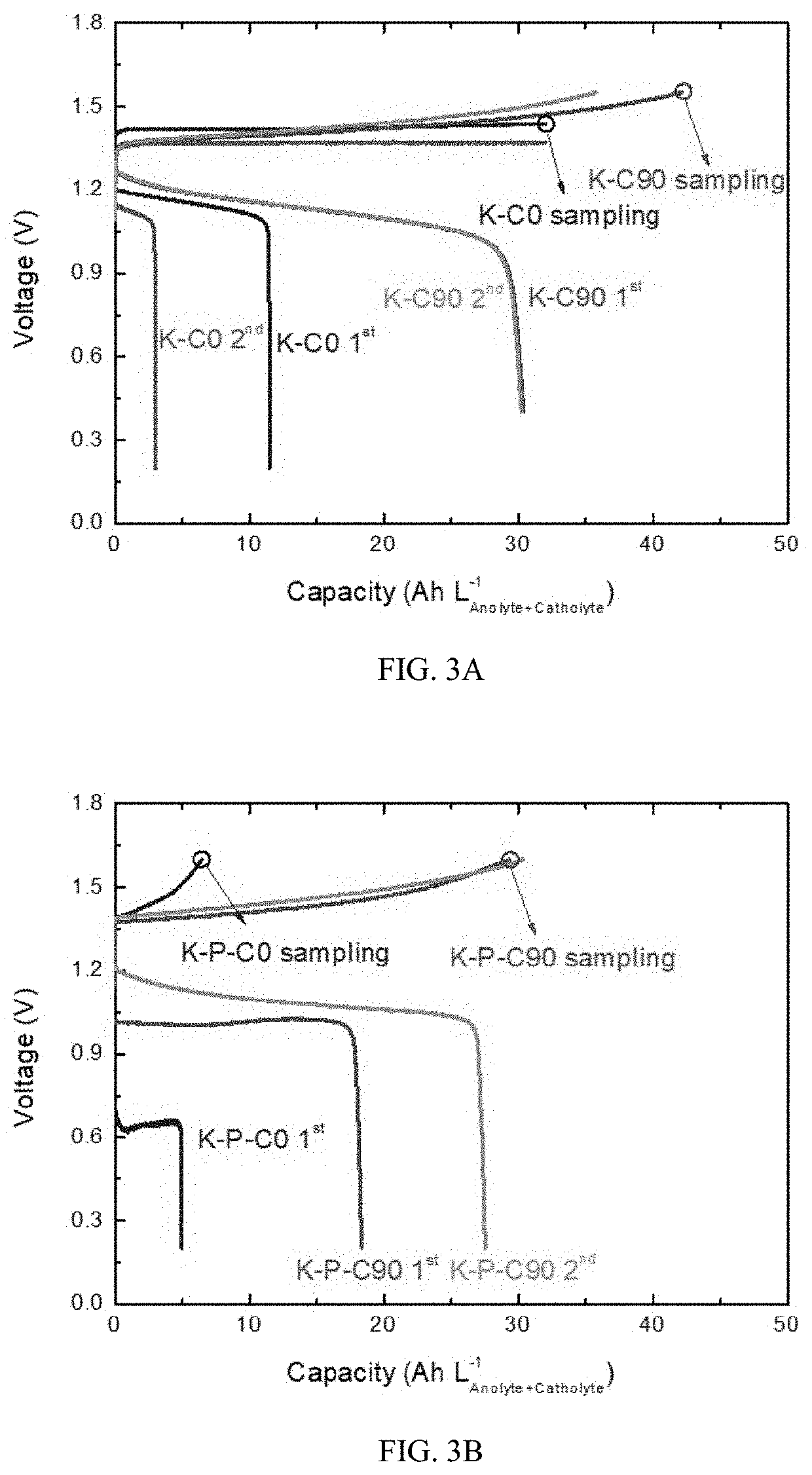
High efficiency zinc-iodine adsorption-aided flow battery with a low cost membrane
ActiveUS20220085401A1Improve Coulombic efficiencyLarge specific surface areaReactant parameters controlOther chemical processesElectrolytic agentPolyethylene glycol
A flow battery system and methods are provided for eliminating crossover issues of active materials in redox flow batteries. A solid adsorbent with large specific surface area is disposed in an electrolyte of at least one half-cell, in contact with the electrolyte. During a charging process, the active material in a charged state is captured and stored on surfaces of the adsorbent, so that concentrations of the active material in the electrolyte in the charged state is reduced and the crossover is inhibited. During a discharging process, the active material is desorbed from the adsorbent to the electrolyte and pumped into the stack for reaction. The flow battery stack can have a microporous membrane separator. The electrolyte of the flow battery includes zinc iodide as active material and polyethylene glycol (PEG) as an additive.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Electrolyte based on zinc iodide and application thereof
InactiveCN1933184AImprove conductivityRight melting pointLight-sensitive devicesPhotovoltaic energy generationSolventOrganic compound
This invention relates to an electrolyte based on ZnI2 with the following formula: ZnI2(L)n+mM+wI2+xG+yCP+zA, in which, ligand L is an organic material coordinated with ZnI2 in following number: 0<=n<=6, the solvent M includes amine, mellow, acid nitrile, aether and ester organic solvents and their mixtures or ionic solution, the mol ratio of which to the ZnI2 is 0<=m<=2000, the mol ratio of I simple substance to ZnI2 is 0<=w<=0.5, the gel G is an organic compound of small molecules or an organic compound of high molecules and the mol ratio of which to the ZnI2 is 0<=x<=1 and that of ceramic powder to ZnI2 is 0<=y<=5 and additive A is a compound capable of improving performance of electrolyte and the ratio to the ZnI2 is 0<=z<=2.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Composite electrode, array substrate and display device
InactiveUS20160126421A1Improve transfer rateReduce sheet resistanceSolid-state devicesNon-linear opticsAluminium chlorideComposite electrode
A composite electrode comprises at least one graphene layer and at least one doping layer, and two adjacent layers are not both the doping layer; wherein the doping layer is an aluminum chloride layer or a zinc iodide layer. It is used for manufacture of a display device.
Owner:BOE TECH GRP CO LTD +1
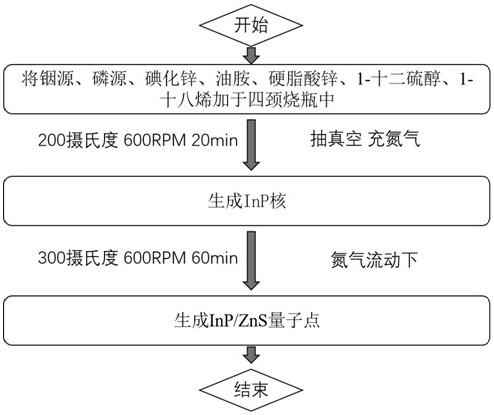
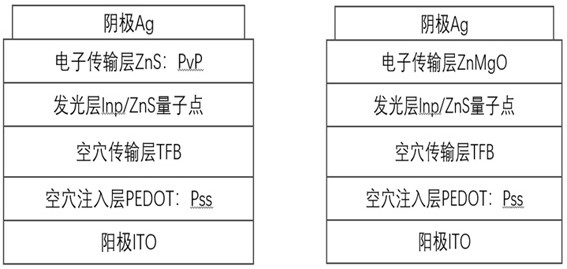
Blue light InP/ZnS quantum dot, preparation method thereof and application of blue light InP/ZnS quantum dot in QLED
ActiveCN112625681ASmall lattice mismatchQuality improvementMaterial nanotechnologySolid-state devicesIndiumFluorescence
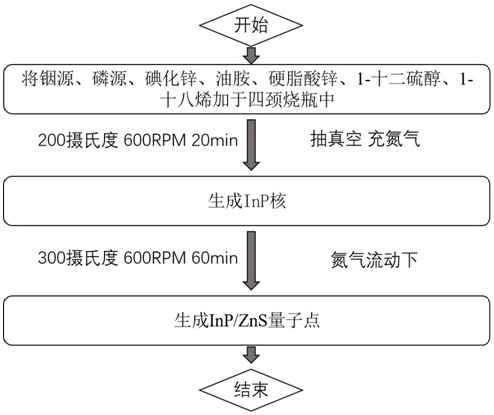
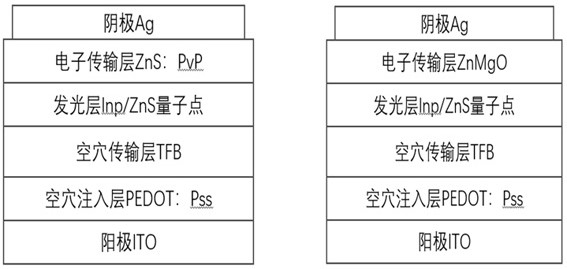
The invention discloses a blue light InP / ZnS quantum dot, a preparation method thereof and application of the blue light InP / ZnS quantum dot in a QLED device. According to the invention, an indium source, a phosphorus source, zinc iodide, oleylamine, zinc stearate, 1-dodecanethiol and 1-octadecene are placed in a 50ml flask, in a nitrogen environment, different temperatures and reaction time are controlled to respectively form an InP core and a ZnS shell, and then the InP / ZnS quantum dot emitting pure blue light is obtained. Compared with the traditional method, the method for synthesizing the InP / ZnS quantum dot by utilizing a one-pot method is simple and more time-saving, the lattice mismatch degree between the shell and the core of the synthesized quantum dot is lower, the defects are fewer, the fluorescence quantum efficiency is higher, the light emitting peak wavelength of the blue quantum dot synthesized by utilizing the traditional method is mostly 470nm or above, the quantum dot disclosed by the invention has a light-emitting peak wavelength of 470 nm or less and is pure blue light, and the prepared QLED has the non-toxic advantage compared with cadmium quantum dots and is more beneficial to commercialization.
Owner:FUZHOU UNIV +1
Low-permeability solid-free completion fluid
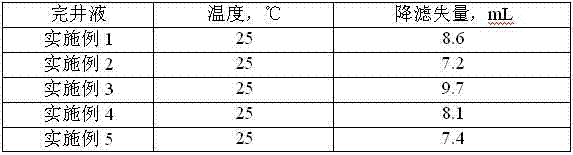
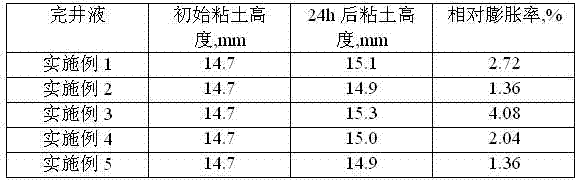
A low-permeability solid-free completion fluid is characterized by comprising the following components in mass percentage: 20-65% of specific gravity regulator, 1-3% of fluid loss agent, 1-2% of antiswelling agent, 0.05-0.1% of crystallization inhibitor, 0.5-1% of anti-collapse agent, 0.5-1% of corrosion inhibitor and the balance of water, wherein the specific gravity regulator is one or a combination of more than two of zinc chloride, zinc bromide, zinc iodide, zinc sulfate, zinc acetate, zinc chlorate and zinc nitrate; the fluid loss agent is carboxymethyl modified starch; the antiswelling agent is diallyl dimethyl ammonium chloride; the crystallization inhibitor is one or a combination of two of nitrilotriacetic acid and EDTA (ethylene diamine tetraacetic acid); the anti-collapse agent is nitro potassium humate; and the corrosion inhibitor is one or a combination of more than two of sodium molybdate, sodium tungstate and sodium chromate. The low-permeability solid-free completion fluid is large in specific weight, good in stability and resistant to high temperature and high pressure, can reduce the swelling rate of clay, improves the core permeability restoring value and does not generate precipitates.
Owner:DAQING HUAYING CHEM IND
Low Power Metal Halide DC Lamp
ActiveCN103456598BStable light outputStable discharge arcGas discharge lamp detailsMetal-halide lampMetal halides
The invention belongs to the technical field of illumination of light sources, and particularly relates to a small-power metal halide direct current lamp which is stable in arc and can work under a direct current stabilizer. The small-power metal halide direct current lamp comprises a quartz bubble shell, wherein a sealed light-emitting arc tube is arranged in the quartz bubble shell, a cathode and an anode are arranged in the sealed light-emitting arc tube, one end of the cathode extends into the light-emitting arc tube, the anode is matched with the cathode, the other end of the cathode and the other end of the anode are located in the quartz bubble shell and are connected with molybdenum sheets respectively, the molybdenum sheets are connected with molybdenum rod leads respectively, the molybdenum rod leads extend out of the quartz bubble shell, the light-emitting arc tube is filled with stuffing, the stuffing comprises 3-6.5 atmospheres of xenon, 0.5-0.9mg of mercury, zinc iodide and one or more of metal halide DyI3, DyBr3, HoBr3, NdI3, CsI, InI, NaI, ScI3, ThI4 and T1I. The small-power metal halide direct current lamp can output stable light rays, bring convenience to using and reduce using cost of users, and is long in service life.
Owner:CHANGZHOU NEWPHENIX LIGHTING MFR
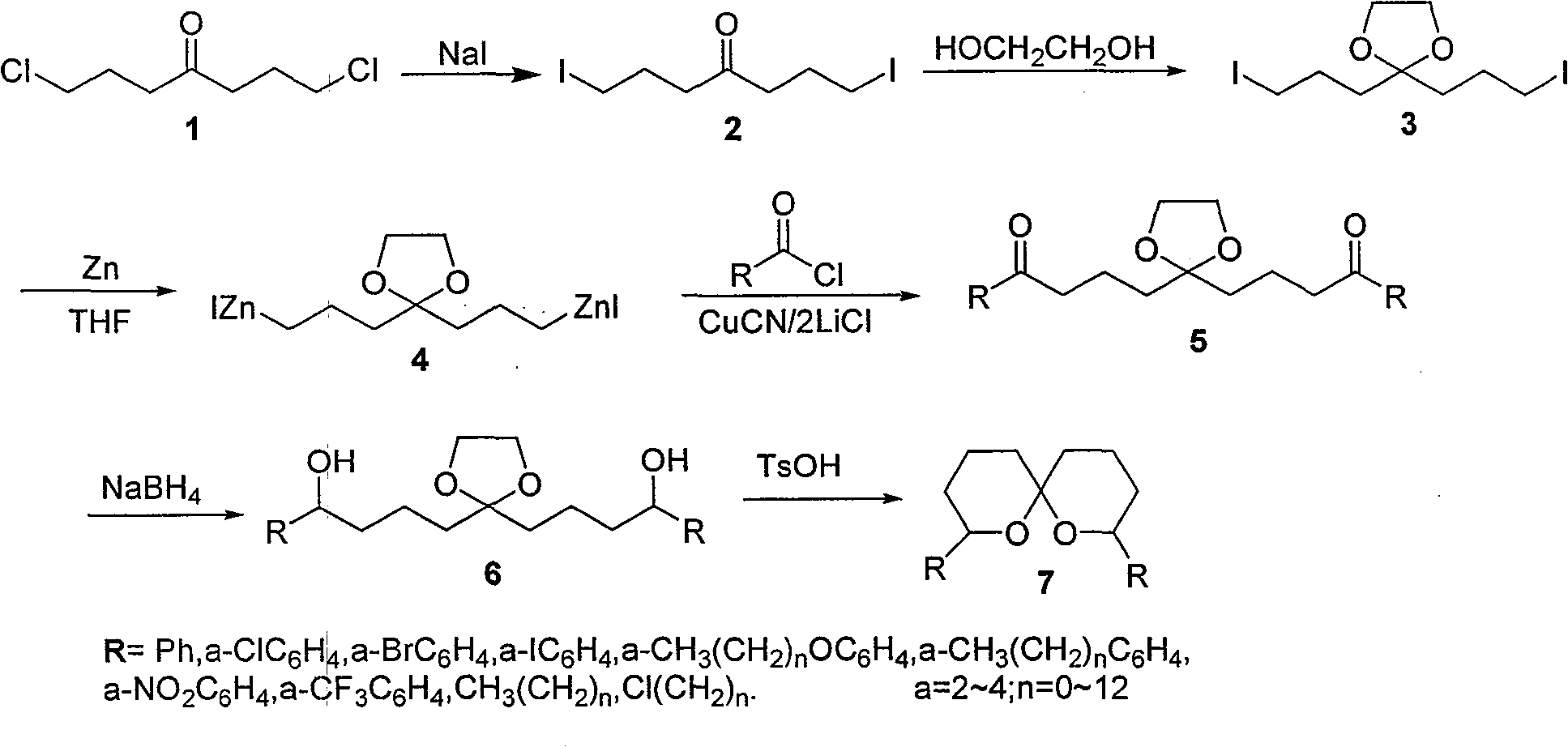
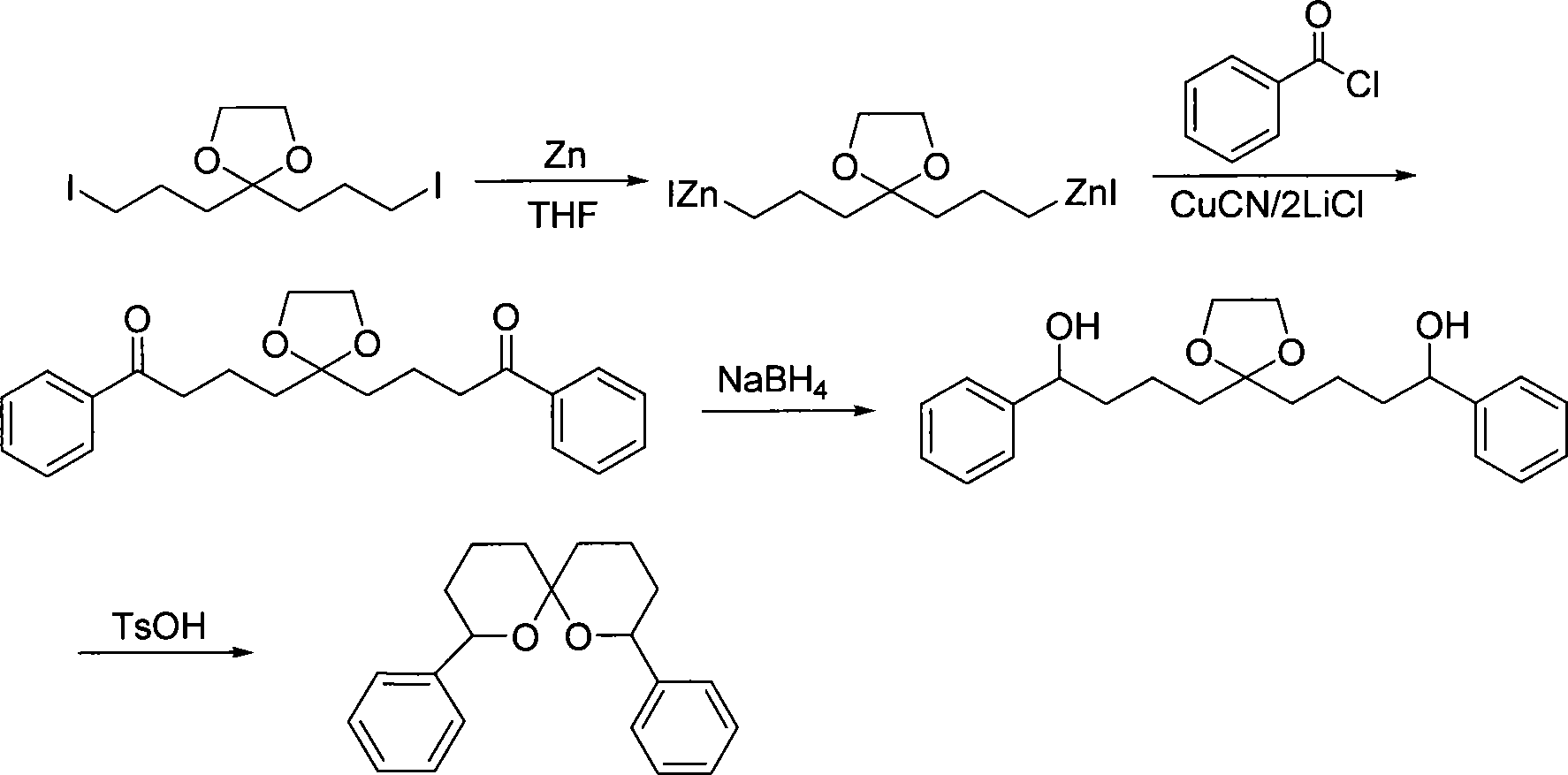
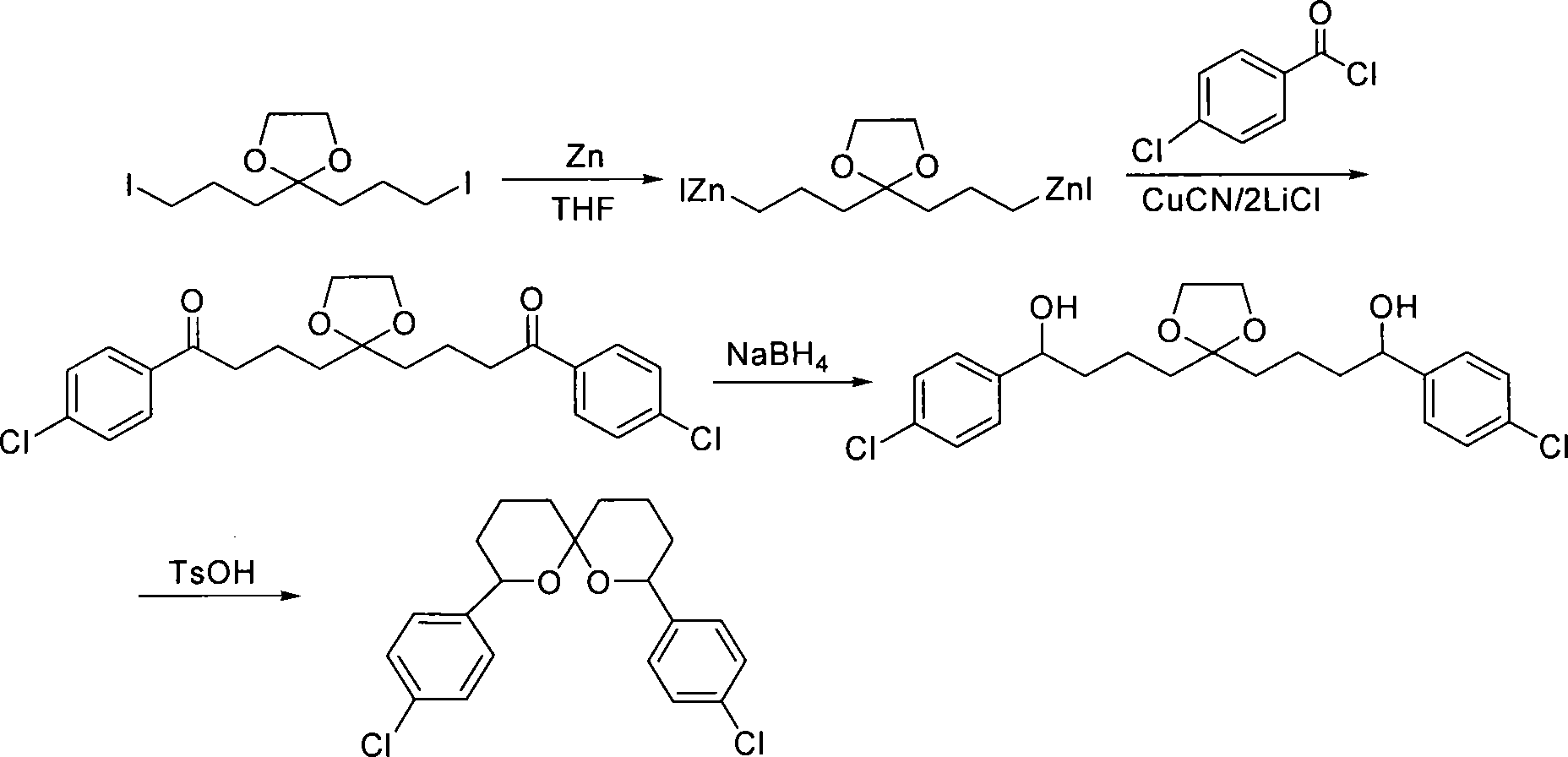
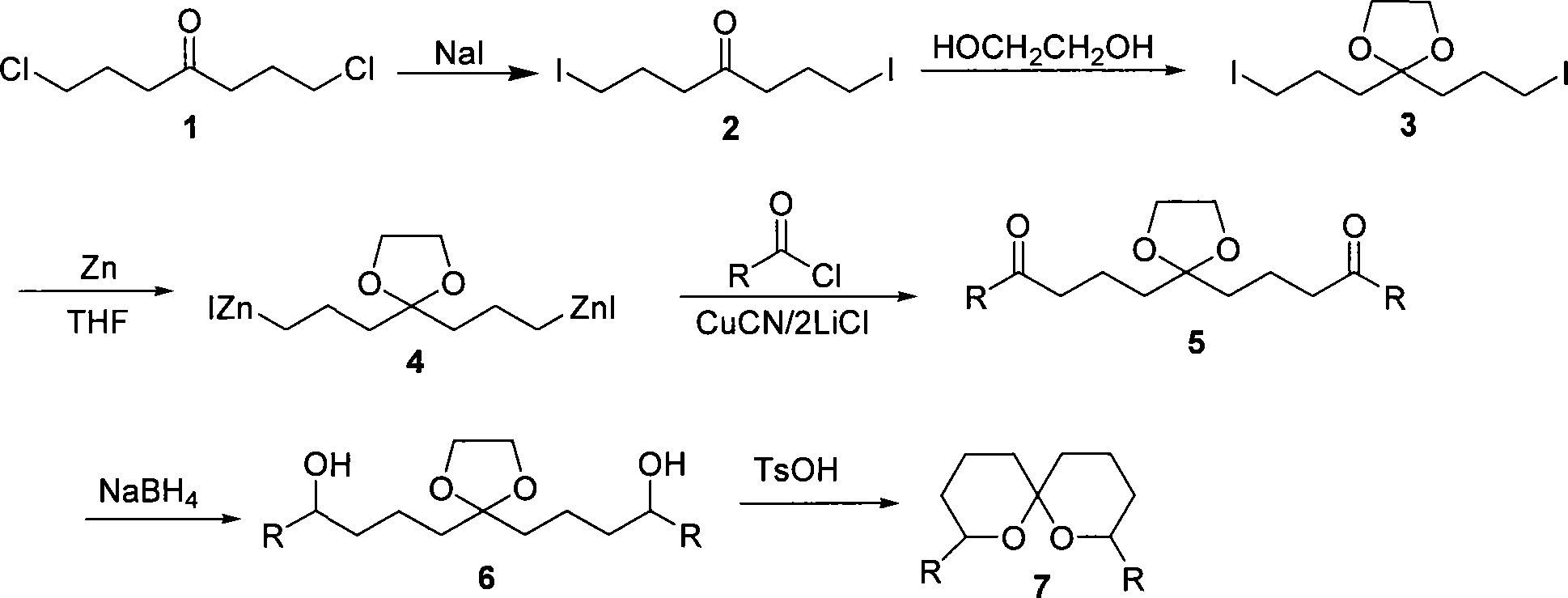
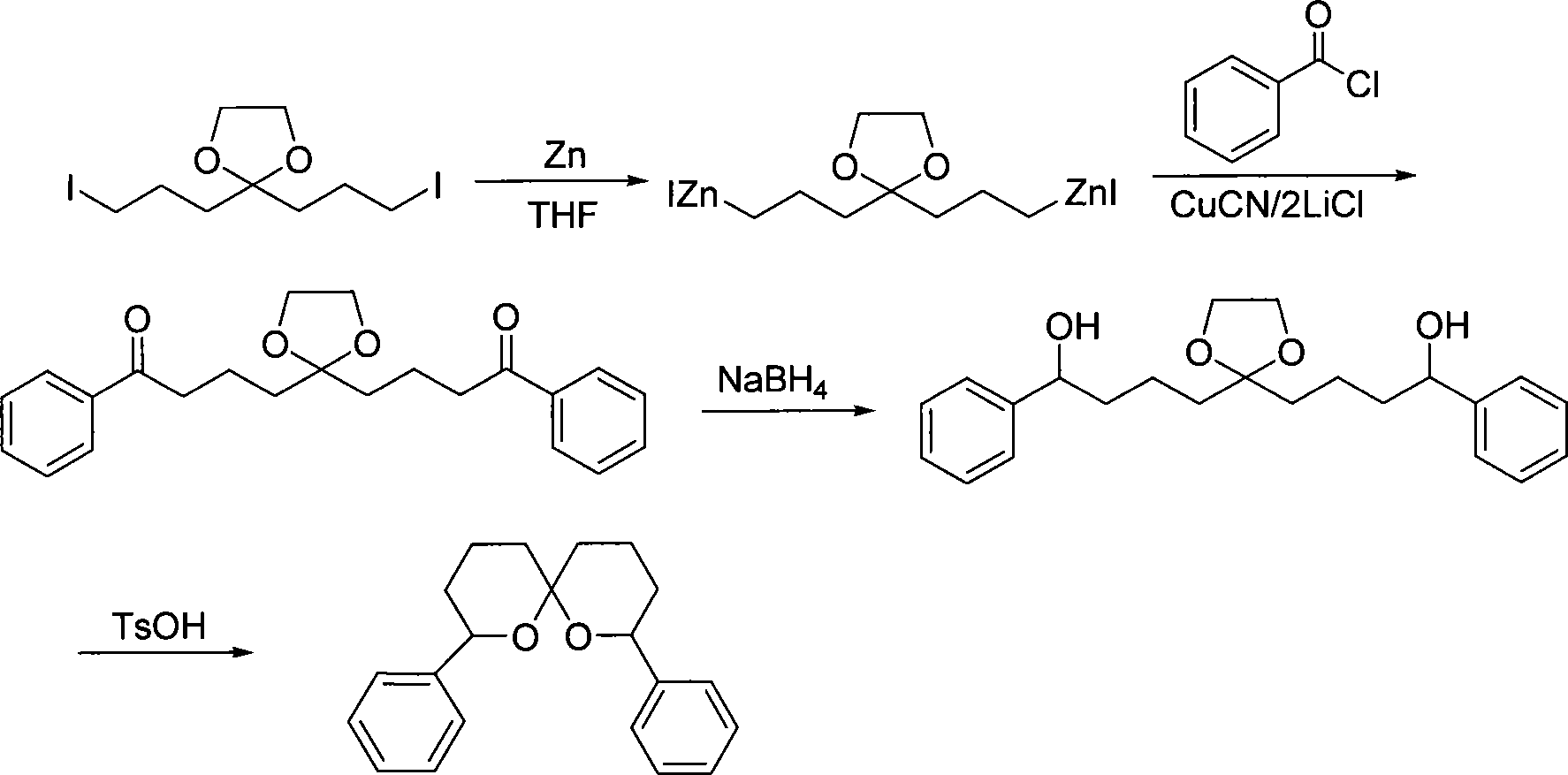
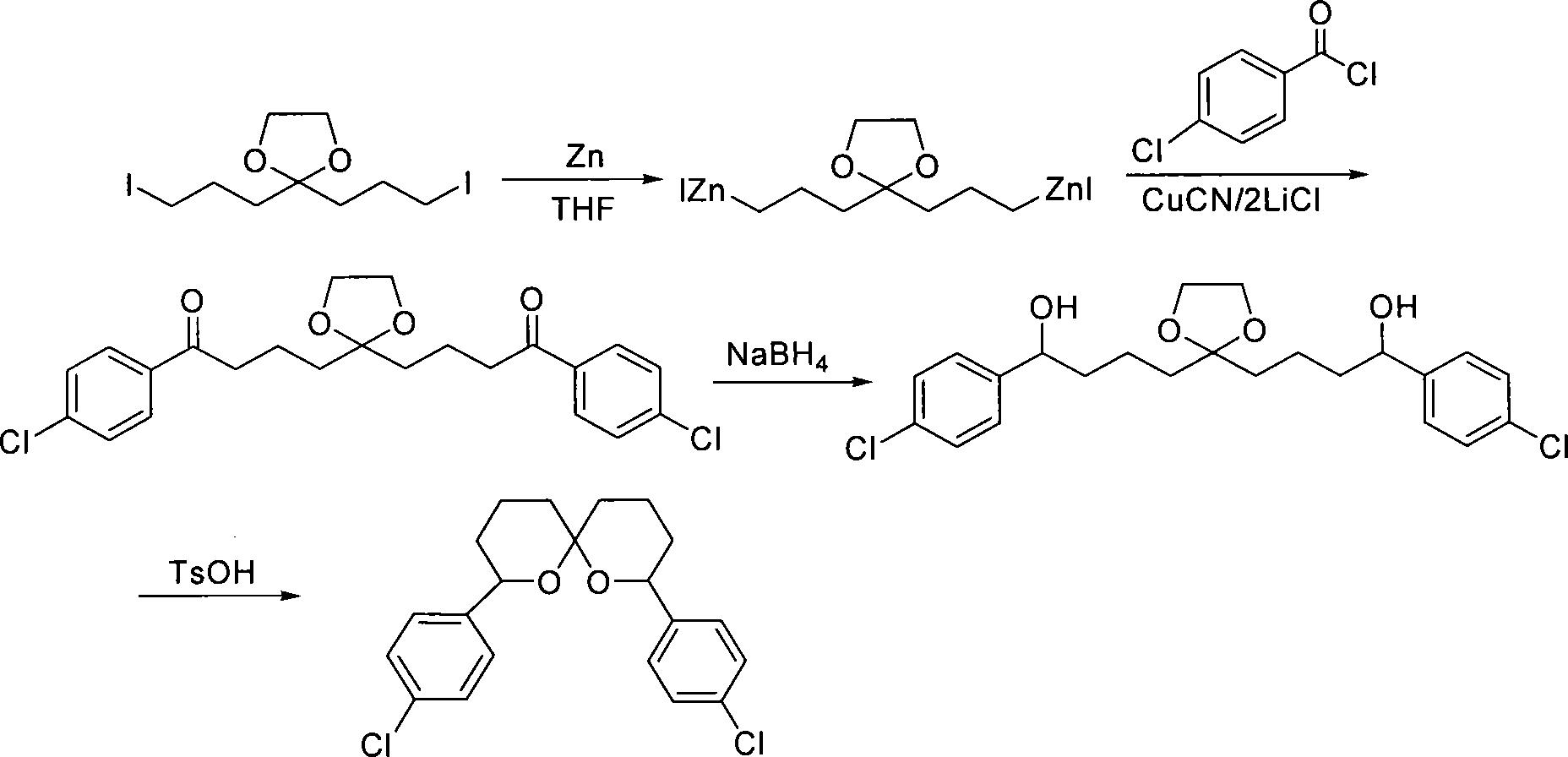
Method for synthesizing spiro ketal by employing double organic zincons
InactiveCN101182324BShort synthesis cycleLower synthesis costOrganic chemistryDiketoneSodium borohydride
The present invention discloses a method of synthesizing spiro ketal by dual-organic zinc reagent. The method is that the dual-organic zinc iodide with ketal is used to react with acyl chloride to produce the diketone with ketal to be deoxidized and synthesized as dihydroxy ketal by sodium borohydride, and then the spiro ketal is further synthesized under the catalysis of p-toluenesulfonic acid. The present invention shortens the synthesis route of the spiro ketal greatly and at the same time reduces the synthesis cost; the reaction yield is high, and the total yield is 24 percent to 38 percent; the reaction condition is gentle; the operation is simple; the present invention is easy for the industrialized production.
Owner:NORTHWEST NORMAL UNIVERSITY
Negative electrode electrolyte for zinc-iron flow battery
PendingCN114551954AReduce generationInhibit growthRegenerative fuel cellsOrganic electrolytesZinc bromideSupporting electrolyte
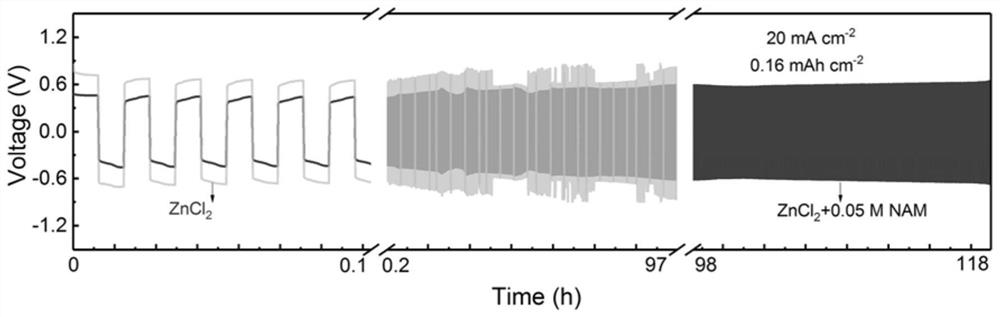
The invention is applied to the field of zinc-iron flow batteries, and particularly relates to a negative electrode electrolyte for a zinc-iron flow battery. An electrolyte in a negative electrode electrolyte of the zinc-iron flow battery comprises a zinc salt, an additive and a supporting electrolyte, the zinc salt is one or two of zinc chloride, zinc bromide, zinc sulfate and zinc iodide, the additive is nicotinamide, and the supporting electrolyte is potassium ions or sodium ions. According to the invention, by selecting the additive and utilizing the complexing structure of divalent zinc ions and the additive and the adsorption effect of a zinc deposition layer and the additive, the growth of zinc dendrites and the generation of byproducts are inhibited, the zinc deposition morphology is improved, the zinc deposition is compact and uniform, the performance of the battery is improved, and the cycle life of the battery is prolonged. The invention has the advantages of outstanding performance, safety, environmental protection, low price and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Direct current supply metal halide lamp
ActiveCN106449351AReduce manufacturing costGood market benefitsGas discharge lamp detailsNoble gasMetal-halide lamp
The present invention discloses a direct current supply metal halide lamp. The lamp comprises an arc tube which is internally filled with noble gas and metal halide, and the metal halide includes zinc iodide and cesium bromide so as to solve the problem that the direct current supply xenon metal halide lamp has scintillation and the jitter phenomenon with different degrees and is low in luminous efficiency. The direct current supply metal halide lamp is low in the price of the xenon metal halide lamp, stable in quality and wide in the market prospect.
Owner:JIAXING GUANGTAI LIGHTING
Metal halide pellet of ceramic metal halide lamp, preparation method for metal halide pellet and mercury-free ceramic metal halide lamp
InactiveCN104658860AImprove luminous efficiencyGood colorGas discharge lamp detailsCold cathode manufactureIodideMercury pollution
The invention provides a metal halide pellet of a ceramic metal halide lamp. The metal halide pellet is made from a complex compound formed by first metal halide and second metal halide; first metal halide is made from composition of thulium iodide, samarium iodide and thallium iodide, and second metal halide is made from zinc iodide; thulium iodide accounts for 50-80% by mass of first metal halide, samarium iodide accounts for 5-10% by mass of first metal halide, and a mass ratio of first metal halide to second metal halide is (1-10):1. The method provides a preparation method for the metal halide pellet of the ceramic metal halide lamp, and the prepared metal halide pellet has the moisture content lower than 1.2 ppm and is good in sphericity and stable in quality. The invention further discloses a mercury-free ceramic metal halide lamp containing the metal halide pellet, and the mercury-free ceramic metal halide lamp not only eliminates harm of mercury pollution but also has the advantages of high light efficiency, high color temperature, good color rendering property and stable working.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Ceramic halogen lamp
InactiveCN104183466AIncrease vapor pressureIncrease steam pressureGas discharge lamp detailsNoble gasSodium iodide
Provided is a ceramic halogen lamp which comprises a ceramic electric arc tube filled with a rare gas, first metal halides, second metal halides, and zinc. The first metal halides comprise sodium iodide, thallium iodide, dysprosium iodide, and thulium iodide. The second metal halides comprise zinc iodide and gallium iodide. The ceramic halogen lamp is environmentally friendly.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Ceramic halogen lamp
InactiveCN104183465AIncrease pipe pressureHigh light efficiencyGas discharge lamp detailsNoble gasSodium iodide
Provided is a ceramic halogen lamp which comprises a ceramic electric arc tube filled with a rare gas, first metal halides, a second metal halide, and zinc. The first metal halides comprise sodium iodide, thallium iodide, dysprosium iodide, and thulium iodide. The second metal halide is zinc iodide. The ceramic halogen lamp is environmentally friendly.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
A blue light inp/zns quantum dot and its preparation method and application in qled
ActiveCN112625681BSmall lattice mismatchQuality improvementMaterial nanotechnologySolid-state devicesIndiumFluorescence
The invention discloses a blue-light InP / ZnS quantum dot and its preparation method and application in QLED devices. The invention combines indium source, phosphorus source, zinc iodide, oleylamine, zinc stearate, Alcohol and 1-octadecene are placed in a 50ml flask, and in a nitrogen environment, different temperatures and reaction times are controlled to form an InP core and a ZnS shell, respectively, to obtain InP / ZnS quantum dots that emit pure blue light. Compared with the traditional method, the present invention uses a one-pot method to synthesize InP / ZnS quantum dots, which is simpler and more time-saving, and the lattice mismatch between the shell and core of the synthesized quantum dots is lower, with fewer defects and higher Fluorescent quantum efficiency, blue quantum dots synthesized by traditional methods mostly have a luminous peak wavelength above 470nm, and the quantum dots of the present invention have a luminous peak wavelength below 470nm, which is pure blue light, and the prepared QLED is non-toxic compared to cadmium-based quantum dots. Advantages, more conducive to commercialization.
Owner:FUZHOU UNIV +1
Supercapacitor material based on biochar-coated [Zn4O(BDBC)3]8 and preparation method thereof
InactiveCN110853939AIncrease capacitance densityIncrease energy densityHybrid capacitor electrodesCapacitanceInternal resistance
The invention relates to the technical field of supercapacitor materials, and discloses a supercapacitor material based on biochar-coated [Zn4O(BDBC)3]8 and a preparation method thereof. The supercapacitor material comprises the following formula materials: biological straw, biphenyl-4, 4'-dicarboxylic acid, and zinc iodide. According to the supercapacitor material based on biochar-coated [Zn4O(BDBC)3]8 and the preparation method thereof, a metal organic framework [Zn4O(BDBC)3]8 itself has very low internal resistance and good electrical conductivity so as to reduce the resistance of migratingmetal ions and charges through an electrode material, and increase the specific capacitance and energy density of the capacitor. The biochar has a large specific area and porosity. The surface of thebiochar has a large number of highly aromatized oxygen-containing groups which form hydrogen bonds with the active oxhydryl on the surface of the [Zn4O(BDBC)3]8 such that the [Zn4O(BDBC)3]8 is evenlydispersed and attached tightly to the pores of the biochar, thereby increasing the contact area between [Zn4O(BDBC)3]8 and an electrolyte, diffusing and mirgrating a large number of metal ions and charges between the electrode material and the electrolyte, and increasing the power density and the energy density of the supercapacitor.
Owner:李建龙
A chemical method for synthesizing copper-zinc iodide ternary wide bandgap compound semiconductor thin film materials
ActiveCN106449367BLow priceMild reaction conditionsSemiconductor/solid-state device manufacturingZinc alloysFilm material
The invention belongs to chemical technical field, in particular to a chemical method for semiconductor film materials of ternary wide bandgap compound of synthesis of copper-zinc iodide. The method is that putting the copper-zinc alloy film into iodine vapor with a temperature of 45 DEG C to 80 DEG C for co-oxidation reaction of the copper-zinc alloy. The reaction time takes 3 to 8 hours. After reacting for certain time, Cu2ZnI4 films can be produced in situ on the surface of substrate, and that is the semiconductor film materials of the synthesis of the copper-zinc iodide. The preparation method does not require organic solvents to participate in the reaction or reaction medium. The crystal is crystallized well. The product can be used directly without complicated processing. The method has the advantages of simple operation, rapid reaction, better environmental protection, low energy consumption, low cost and good reproducibility. In addition, a film can be directly formed on the substrate surface in the method. The material is more beneficial for the application in photoelectric conversion devices.
Owner:XUCHANG UNIV
Method for synthesizing spiro ketal by employing double organic zincons
InactiveCN101182324AShort synthesis cycleLower synthesis costOrganic chemistryDiketoneSodium borohydride
The present invention discloses a method of synthesizing spiro ketal by dual-organic zinc reagent. The method is that the dual-organic zinc iodide with ketal is used to react with acyl chloride to produce the diketone with ketal to be deoxidized and synthesized as dihydroxy ketal by sodium borohydride, and then the spiro ketal is further synthesized under the catalysis of p-toluenesulfonic acid. The present invention shortens the synthesis route of the spiro ketal greatly and at the same time reduces the synthesis cost; the reaction yield is high, and the total yield is 24 percent to 38 percent; the reaction condition is gentle; the operation is simple; the present invention is easy for the industrialized production.
Owner:NORTHWEST NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com