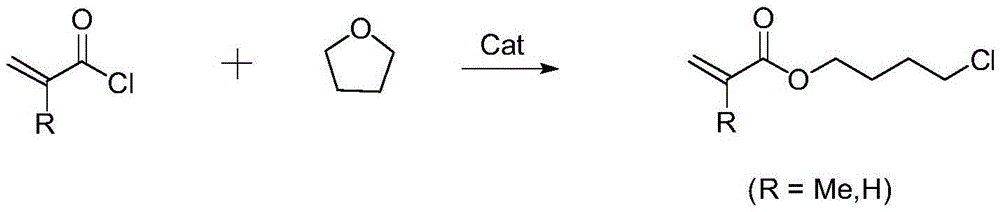
Preparation method of acrylic acid-4-chlorobutyl ester compounds
A compound and chlorobutyl ester technology, applied in the field of preparation of acrylic acid-4-chlorobutyl ester compounds, can solve the problems of high price, inability to recycle, application limitation and the like, and achieve the effects of mild preparation reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Into a 1000ml four-necked round-bottomed flask equipped with mechanical stirring, thermometer and reflux condenser, add 173g tetrahydrofuran, 200g toluene, 10.9g zinc chloride, stir, heat up to 65°C, add 209g methacryloyl chloride dropwise, react for 5h . Gas chromatography detection (analysis conditions are: nitrogen as carrier gas, flow rate of 1.5mL / min., injection volume of 0.2 microliters; FID hydrogen flame ion detector, SEG30QC3 / AC5 column, gasification chamber temperature 280 ℃.) The reaction results showed that the raw material methacryloyl chloride was completely reacted, and the yield of the target product-4-chlorobutyl methacrylate reached 91.2%.
Embodiment 2
[0019] To a 1000ml four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and a reflux condenser, add 216.3g of tetrahydrofuran and 22.5g of zinc bromide, stir, raise the temperature to 50°C, add 181g of acryloyl chloride dropwise, and react for 8h. The gas chromatography detection reaction results showed that the raw material acryloyl chloride was completely reacted, and the yield of the target product acrylic acid-4-chlorobutyl ester reached 94.5%.
Embodiment 3
[0021] To a 1000ml four-necked round-bottomed flask equipped with mechanical stirring, a thermometer and a reflux condenser, add 165.2g of tetrahydrofuran and 4.4g of zinc chloride, stir, raise the temperature to 60°C, add 135.8g of acryloyl chloride dropwise, and react for 4h. The gas chromatography detection reaction results showed that the raw material acryloyl chloride was completely reacted, and the yield of the target product 4-chlorobutyl acrylate reached 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com