Preparation method of thioester compounds
A compound and thioester technology, applied in the fields of organic chemistry, chemical instruments and methods, organic chemistry, etc., can solve problems such as unpleasant smell and catalyst poisoning, and achieve strong designability, easy operation and good reaction applicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The present invention will be further described below in conjunction with specific embodiments.
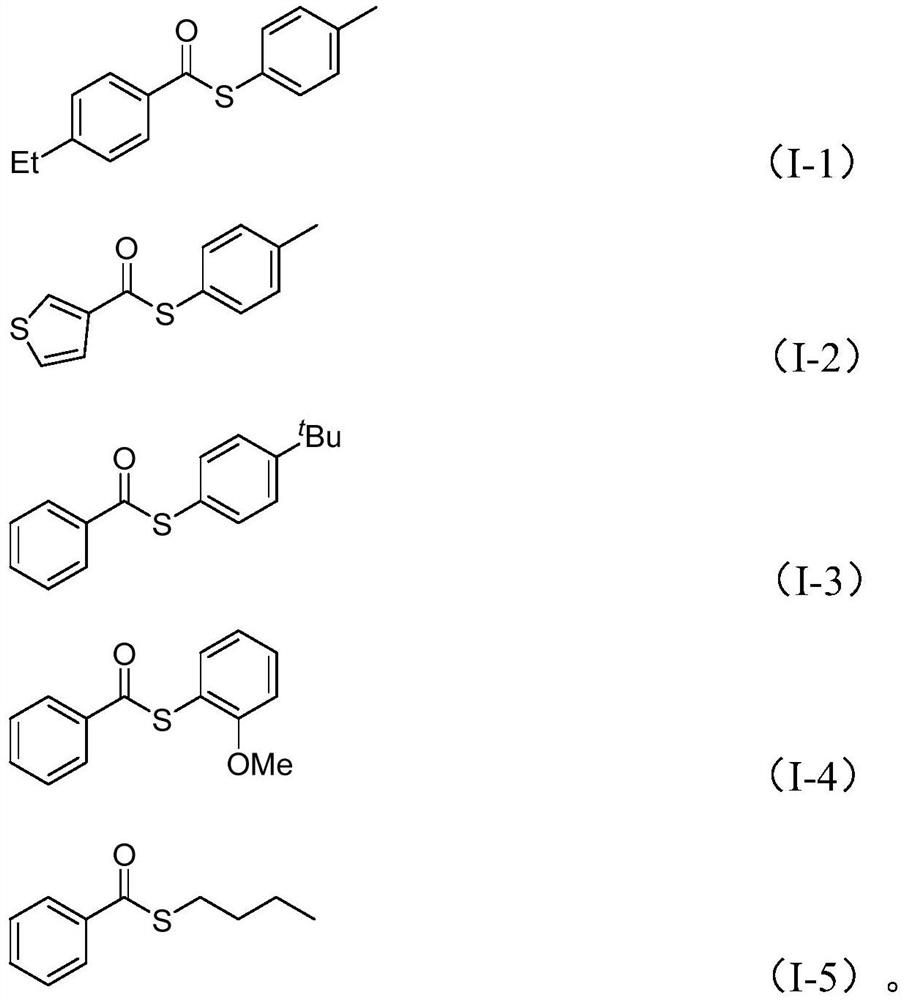
[0036] Add nickel trifluoromethanesulfonate, 4,4'-di-tert-butyl-2,2'-bipyridine, molybdenum carbonyl, potassium carbonate, zinc iodide, water , arylboronic acid (II), sulfonyl chloride (III) and organic solvent 2mL, mixed and stirred evenly, according to the reaction conditions in Table 2, the reaction was completed for 16 hours, filtered, silica gel sample mixing, and purified by column chromatography to obtain the corresponding thioester compound (I), the reaction process is shown in the following formula:
[0037]
[0038] The raw material addition of table 1 embodiment 1~15
[0039]
[0040] Table 2
[0041]
[0042] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Ph is phenyl, Me is methyl, Et is ethyl, OMe is methoxy, t-Bu is tert-butyl, Bu is butyl , i-Pr is isopropyl, Cy is cyclohexyl, and NMP is N-methylpyrrolidone.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com