Method for synthesizing spiro ketal by employing double organic zincons
A technology of spiro ketal and organozinc, which is applied in the synthesis of spiro ketal, using double organozinc reagents in the field of spiro ketal synthesis, achieving the effects of high yield, shortened synthesis cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
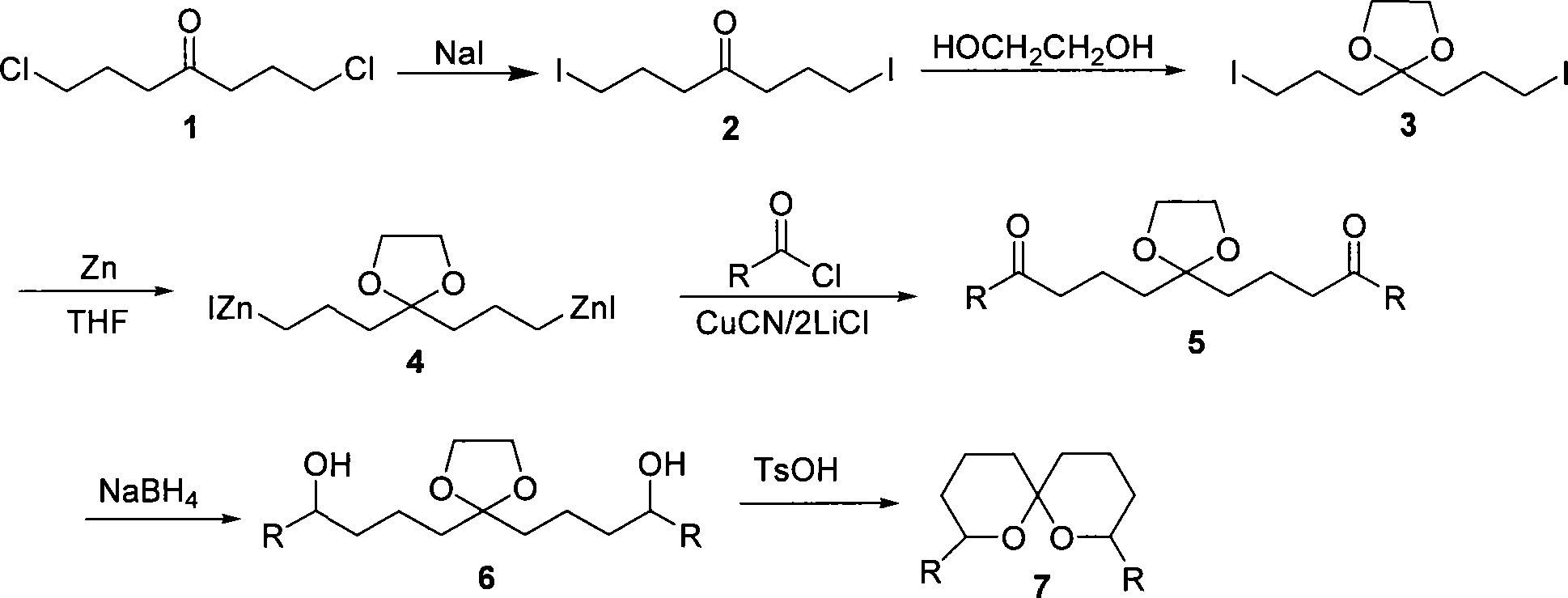
[0031] Embodiment 1, reaction equation is as follows:
[0032]
[0033] 1. Preparation of double organozinc reagents with ketal
[0034] Add dry NaI (0.6mol), dry 100ml acetone and 1,7-dichloro-4-heptanone (compound 1) (0.2mol) into a single-necked flask, and install a reflux condenser to reflux for 8h. After cooling, evaporate the acetone, then add 100ml of ether to dissolve the product, filter off NaCl and excess NaI, and evaporate the solvent to obtain the crude product. Finally, recrystallization with methanol gave white crystals (compound 2) (mp: 41-42°C). The yield was 99%.
[0035] Then add 1,7-diiodo-4-heptanone (0.025mol), ethylene glycol (0.03mol), p-toluenesulfonic acid (0.001mol) and 60ml benzene in a single-necked flask, and then install the water separator and reflux Condenser, fill the water separator with benzene, heat to reflux until about 0.5ml of water is separated, it takes about 2 hours. Stop heating, add 0.3g of potassium carbonate and stir for 2h ...
Embodiment 2
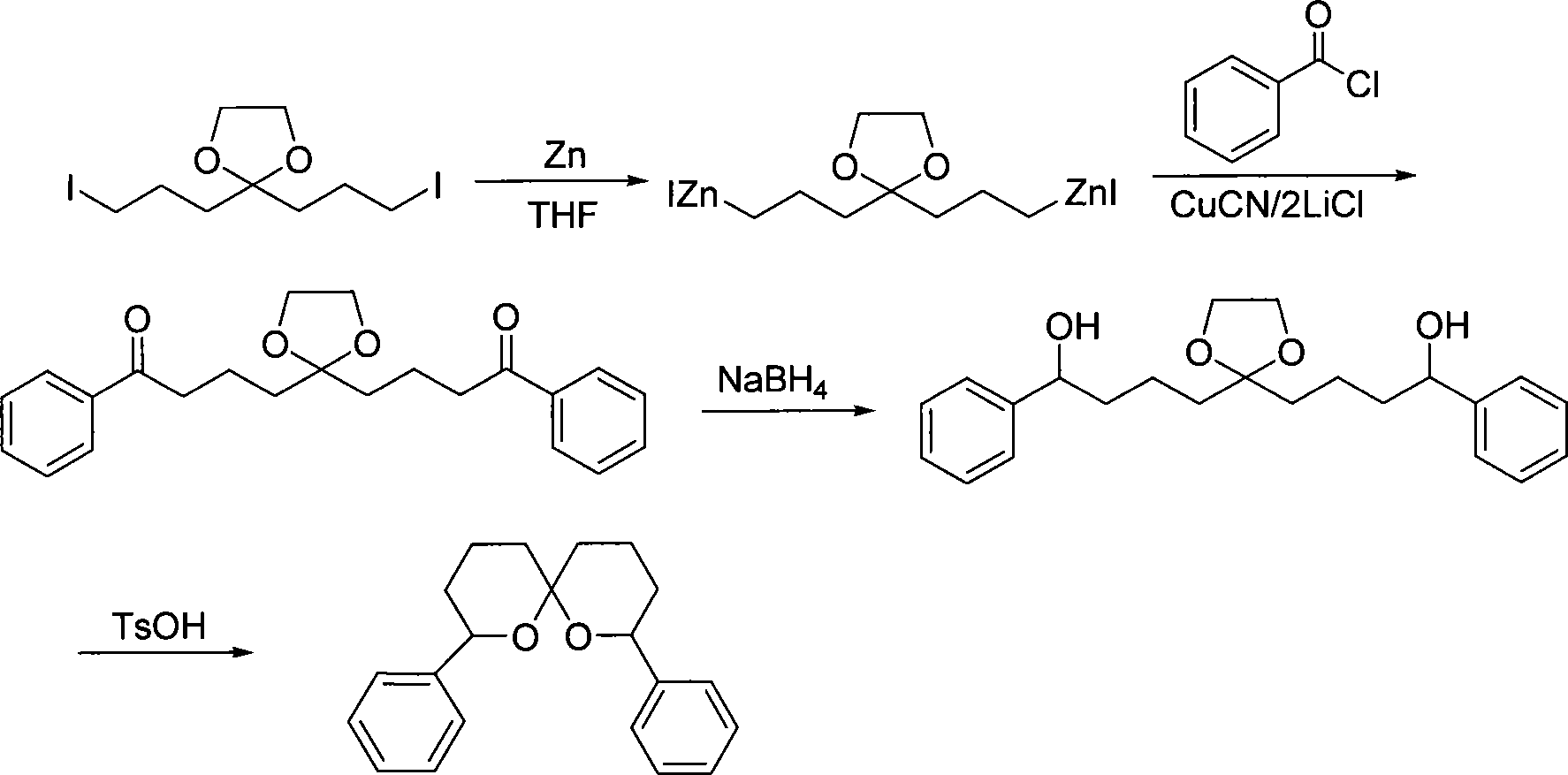
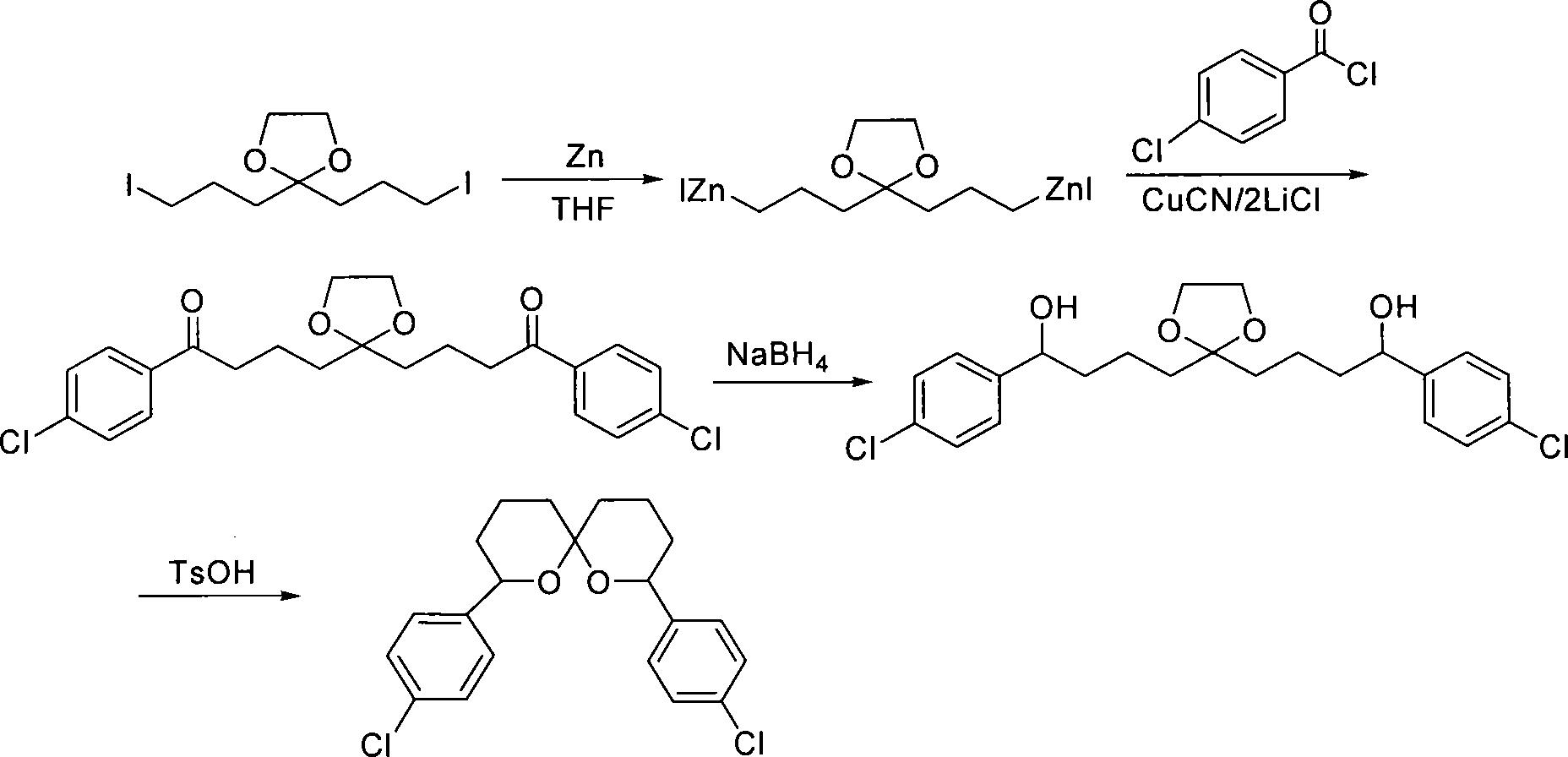
[0047] Embodiment 2, reaction equation are as follows:
[0048]
[0049] 1. Preparation of double organozinc reagents with ketal
[0050] The preparation method is the same as in Example 1.
[0051] 2. Synthesis of diketone compound 5
[0052] Add zinc powder (0.11mol, 7.1g) into a reactor, protect it with argon, add 10mlTHF, stir, then add 1ml1,2-dibromoethane, heat it to reflux until foaming, cool to room temperature and add 1ml of trimethylchlorosilane (1,2-dibromoethane and trimethylchlorosilane are used to activate zinc powder), after stirring for 15min, a solution of compound 3 (0.05mol) in THF (80ml) was added. Heat it to keep the temperature at 35-40°C, and after about 3 hours, the zinc powder disappears to obtain a THF solution of compound 4, which is cooled to room temperature for later use.
[0053] Add dry CuCN (0.1mol) and dry LiCl (0.2mol) to another reactor, protect it with argon, add 80ml THF, stir, wait for it to dissolve and cool to -30°C, slowly add th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com