The preparation method of zinc citrate ammonia
A technology of zinc citrate ammonia and citric acid, which is applied in the field of preparation of zinc citrate ammonia, can solve problems such as difficult release, limited duration of inorganic salt fertilizer effect, high chelation strength of EDTA chelate salt, etc., and achieve low raw material cost, Good compatibility and good nutrient absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
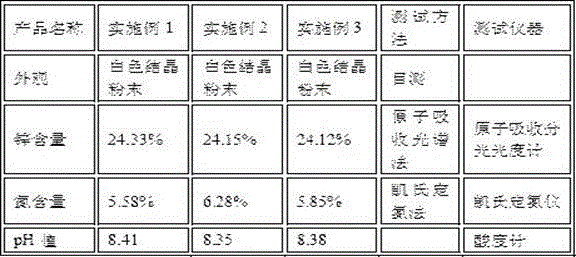
Embodiment 1
[0026] First, accurately weigh 410g of zinc oxide powder, 700g of citric acid monohydrate, 1500ml of ammonia water, and 4000ml of water;
[0027] The preparation method is:
[0028] In the first step, add 4000 ml of water to the reaction kettle, add 410g of zinc oxide powder into the water, stir to dissolve, and disperse evenly;
[0029] In the second step, add citric acid monohydrate 700g in the zinc oxide solution obtained in the first step, and continue to stir until the solution becomes transparent;
[0030] The third step is to start stirring, and slowly add 1125 ml of ammonia water to the transparent solution, and then continue to stir for 0.5h after adding the ammonia water;
[0031] The fourth step is to boil the solution obtained in the third step, concentrate to 1 / 2 of the original volume, stop heating, and naturally cool to room temperature, continue to slowly add the remaining 375ml of ammonia water, and stir for 0.5h;
[0032] In the fifth step, the solution in ...
Embodiment 2
[0034] First, accurately weigh 400g of zinc oxide powder, 680g of citric acid monohydrate, 1400ml of ammonia water, and 4000ml of water;
[0035] The preparation method is:
[0036] In the first step, add 4000 ml of water to the reaction kettle, add 400 g of zinc oxide powder into the water, stir to dissolve, and disperse evenly;
[0037] In the second step, add citric acid monohydrate 680g in the zinc oxide solution prepared in the first step, and continue to stir until the solution becomes transparent;
[0038] The third step is to start stirring, and slowly add 1050 ml of ammonia water to the transparent solution, and then continue to stir for 0.5h after adding the ammonia water;
[0039] The fourth step is to boil the solution obtained in the third step, concentrate to 1 / 2 of the original volume, stop heating, and naturally cool to room temperature, continue to slowly add the remaining 350ml of ammonia water, and stir for 0.5h;
[0040] In the fifth step, the solution in...
Embodiment 3
[0042] First, accurately weigh 440g of zinc oxide powder, 720g of citric acid monohydrate, 1400ml of ammonia water, and 4000ml of water;
[0043] The preparation method is:
[0044] In the first step, add 4000 ml of water to the reaction kettle, add 440g of zinc oxide powder into the water, stir to dissolve, and disperse evenly;
[0045] Second step, in the zinc oxide solution that the first step makes, add monohydrate citric acid 720g, continue to stir until solution becomes transparent;
[0046] The third step is to start stirring, and slowly add 1200 ml of ammonia water to the transparent solution, and then continue to stir for 0.5h after adding the ammonia water;
[0047] The fourth step is to boil the solution obtained in the third step, concentrate to 1 / 2 of the original volume, stop heating, and naturally cool to room temperature, continue to slowly add the remaining 400ml of ammonia water, and stir for 0.5h;
[0048] In the fifth step, the solution in the fourth step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com