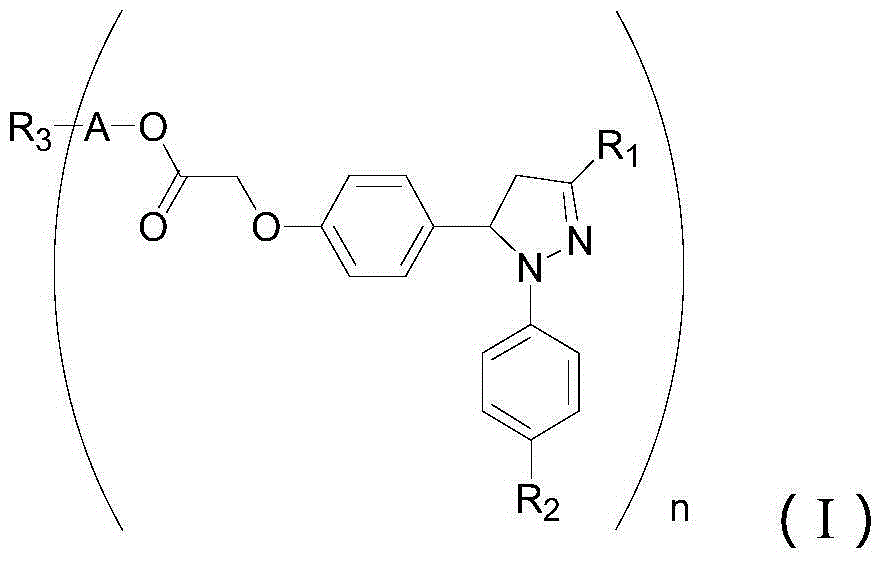
Pyrazoline sensitizer as well as preparation method and application thereof
A technology of pyrazoline and sensitizer, which is applied in the field of pyrazoline sensitizer and its preparation, and can solve the problems of poor sensitivity enhancement effect and unsatisfactory solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
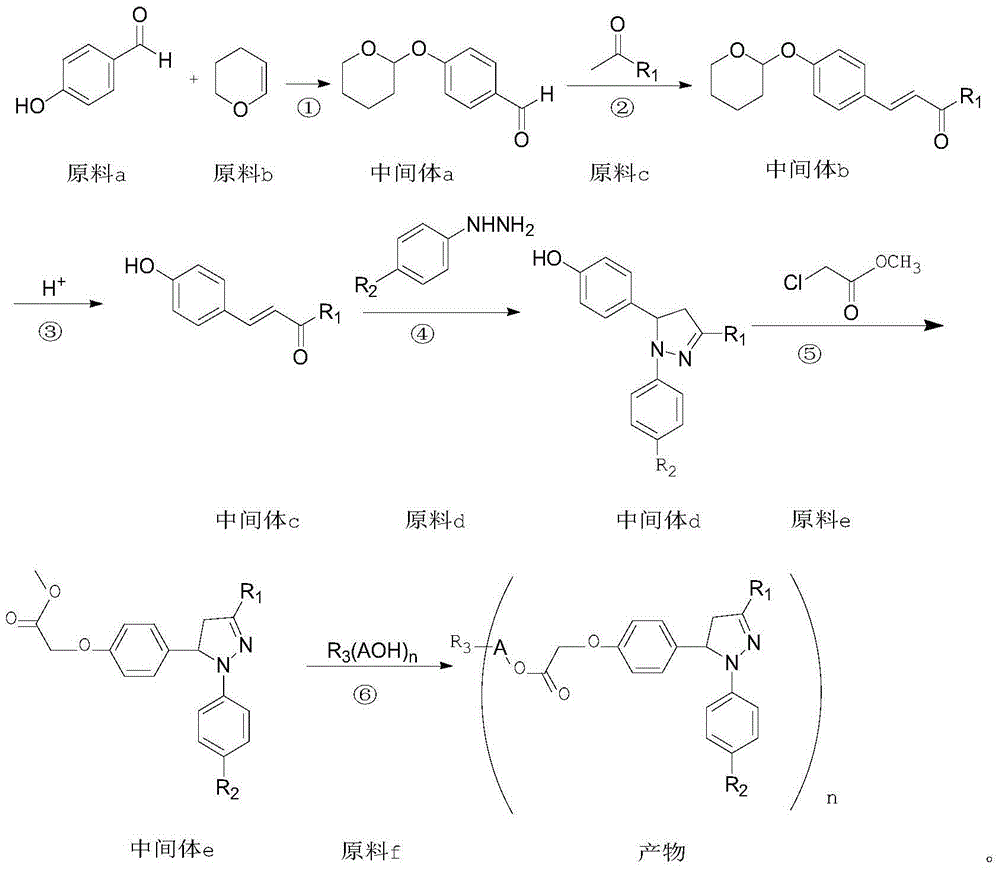
Examples
Embodiment 1
[0041] (1) Preparation of intermediate a:
[0042]
[0043] Add 183g of p-hydroxybenzaldehyde, 5g of catalyst pyridinium p-toluenesulfonate, and 300mL of dichloromethane into a 1000mL four-necked flask, and add 126g of dihydropyran dropwise at a temperature of 50°C for about 1 hour. Stir for 10 h, follow the reaction until the liquid phase does not change and close the reaction, and then remove the dichloromethane by rotary evaporation to obtain 275 g of intermediate a with a purity of 98%, which is directly used in the next reaction;
[0044] (2) Preparation of intermediate 1b:
[0045]
[0046] Add 247g of intermediate a, 235g of benzophenone as raw material c, and 300mL of methanol into a 1000mL four-neck flask, stir in a water bath at room temperature, add 80g of 40% sodium hydroxide solution dropwise within 2h, continue stirring for 6h, and follow the reaction in the liquid phase until no Change again, filter, methanol recrystallization, dry to obtain solid 415g, nam...
Embodiment 2
[0061] The synthesis of intermediate 2e was carried out with reference to Example 1.
[0062] Preparation of product 2:
[0063]
[0064] Add 7.36g of compound 2e, 27.2g of raw material 2f(9-EO), 0.1g of tetraisopropyl titanate, 0.1g of p-hydroxyanisole, and 100mL of xylene into a 500mL four-neck flask, and control the temperature at 90-100°C Heat and stir, distill out the methanol produced by the reaction while heating, and add xylene in time until no methanol is distilled out, filter while it is hot, carry out vacuum distillation on the obtained filtrate, distill out toluene until completely, and obtain a light yellow viscous The thick product is the product 2.
Embodiment 3-12
[0066] Referring to the method of Example 1, the product 3-13 with the following structure is synthesized:
[0067]
[0068]
[0069]
[0070] performance evaluation
[0071] By preparing an exemplary photocurable composition (ie, a photosensitive resin composition), the application performance of the sensitizer represented by formula (I) of the present invention is evaluated.
[0072] 1. Preparation of performance evaluation objects
[0073]
[0074] Fully stir and mix the photosensitive resin composition and PGMEA (propylene glycol methyl ether acetate) with the composition shown in Table 1, and use a bar to coat the surface of a 19 μm thick polyethylene terephthalate film as a support Evenly coated on the surface, and then dried in a dryer at 95° C. for 4 minutes to form a photosensitive resin layer with a thickness of 40 μm.
[0075] Next, a polyethylene film having a thickness of 23 μm was bonded as a protective layer on the surface of the photosensitive re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com