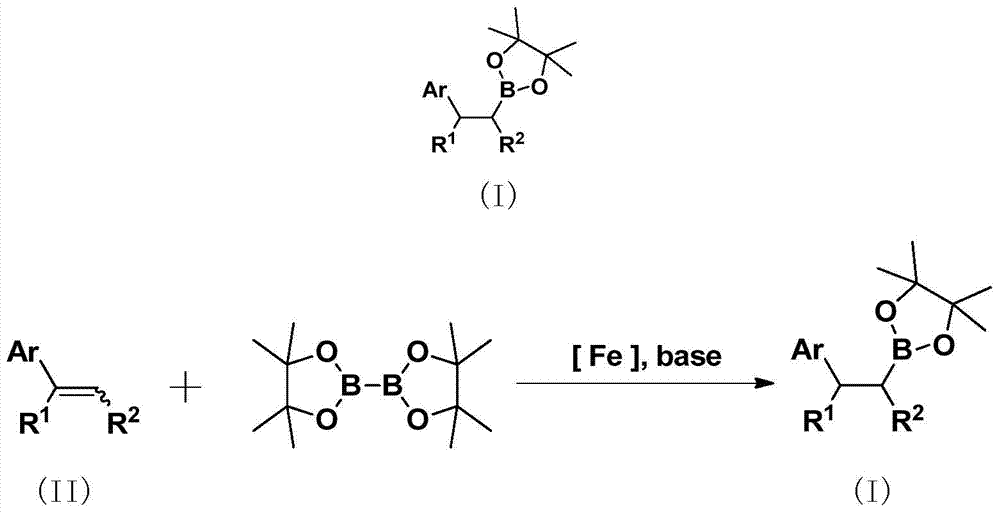
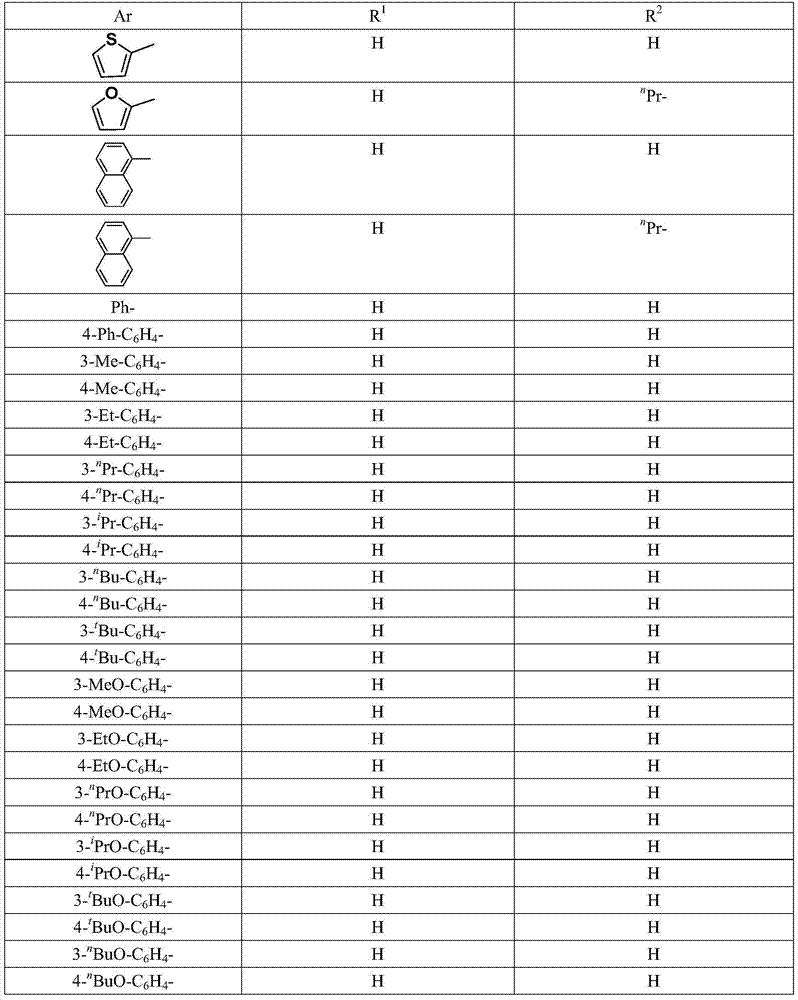
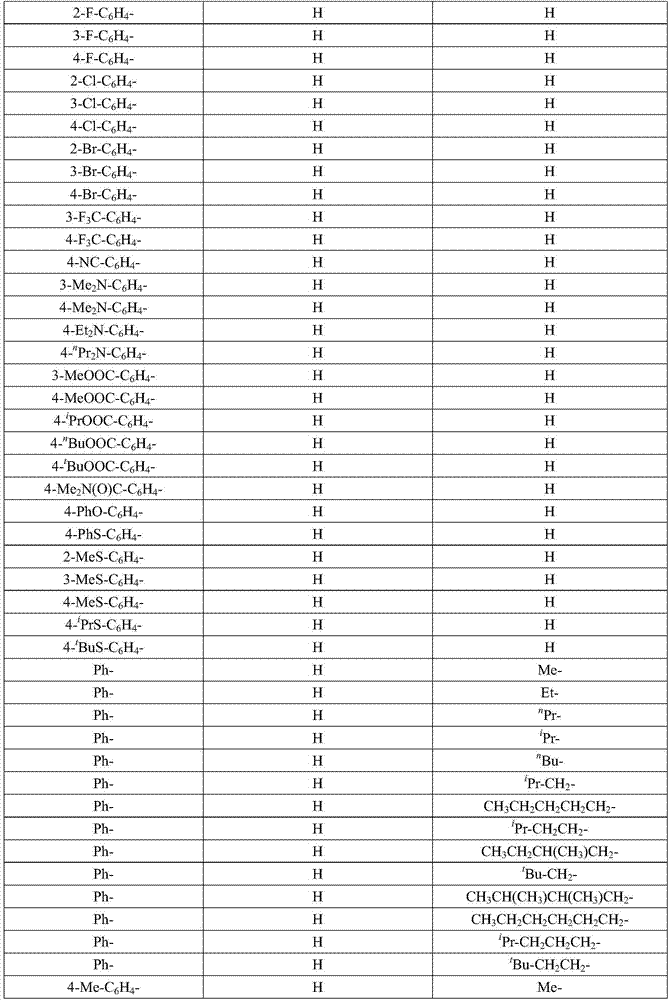
Method for preparing alkyl borate compound based on iron catalysis
An alkyl boronate and catalytic preparation technology, which is applied in the field of preparing alkyl borate compounds based on iron catalysis, can solve the problems of high price, harsh reaction conditions, narrow adaptability of functional groups of substrates and the like, and achieves convenient application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of 2-phenylethyl-1-boronic acid pinacol ester (compound 1)
[0047] Add the catalyst FeCl to the 50ml Schlenk bottle protected by argon at room temperature 2 2.6mg (0.02mmol, 1% of the molar weight of styrene), 20 mL of anhydrous THF, 208 mg (2 mmol) of styrene, 762 mg (3 mmol) of pinacol ester of 1.5 times the molar weight of styrene, 762 mg (3 mmol) of the molar weight of styrene 269 mg (2.4 mmol) of 1.2 times potassium tert-butoxide and 150 mg (2 mmol) of 1 times equivalent tert-butanol. The reaction system is a light brownish yellow turbid liquid, which was reacted at 65°C for 12 hours. After treatment, the solvent was removed by a rotary evaporator, and 50 mL of water was added, extracted with ethyl acetate (4×20 mL), and the combined organic phases were washed with saturated brine (2×30 mL) and washed with anhydrous Na 2 SO 4 After drying, the target compound was obtained by column chromatography, the filler was silica gel, the eluent was...
Embodiment 2
[0048] Example 2 Preparation of 2-(4-methylphenyl)ethyl-1-boronic acid pinacol ester (compound 2)
[0049] Except that the styrene in Example 1 was replaced by the same molar amount of 4-methylstyrene, the same method as in Example 1 was carried out to obtain the separation yield of the target compound of 86%.
Embodiment 3
[0050] Example 3 Preparation of 2-(4-methoxyphenyl)ethyl-1-boronic acid pinacol ester (compound 3)
[0051] Except that the styrene in Example 1 was replaced by the same molar amount of 4-methoxystyrene, the method was the same as in Example 1, and the separation yield of the target compound was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com