Anti-tnfα fully human monoclonal antibody and its application
A fully human antibody, antibody technology, applied in applications, antibodies, anti-animal/human immunoglobulins, etc., can solve problems such as infeasible immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of hybridoma and carrier
[0071] 1. Antigen and lymphocyte preparation and immunization
[0072] 1.1 Antigen TNFα protein preparation
[0073] Human TNFα protein (drug target) and monkey TNFα (drug target) protein were purchased from Yiqiao Shenzhou, diluted with PBS, filtered with a 0.22 μm needle sterile filter, and distributed in 1.5ml cell cryopreservation tubes in- Store at 80°C.
[0074] 1.2 Human lymphocyte preparation
[0075]Fresh tissue samples of chronic tonsillitis were collected aseptically (in cooperation with the Department of Otolaryngology, Affiliated Hospital of Xuzhou Medical College, tonsils were taken from patients undergoing tonsillectomy, 10 patients, aged 5-10 years), and lymphocyte separation medium (GE) Lymphocytes were separated, and 30×10 6 pcs; Aseptically extract human fresh peripheral blood cells, separate the lymphocytes in them with lymphocyte separation medium (GE), take 30×10 6 indivual.
[0076] ...
Embodiment 2
[0109] Example 2: Preparation and Identification of Monoclonal Antibody
[0110] 1. Preparation of antibodies by in vitro culture method
[0111] The 1.1.71, 1.5.58, 1.6.17, 1.24.2, 2.2.108, 2.39.45, 3.85.3, 3.4.32, 3.12.78, 3.76.109 cells, gradually reduce serum to serum-free or serum-free medium containing only a small amount of serum (Invitrogen, 12338-026), put in 225cm 2 Square bottle, according to inoculation 1×10 5 cells / ml, inoculate 100ml, and inoculate 4 bottles of each strain of cells.
[0112] 2. Antibody purification
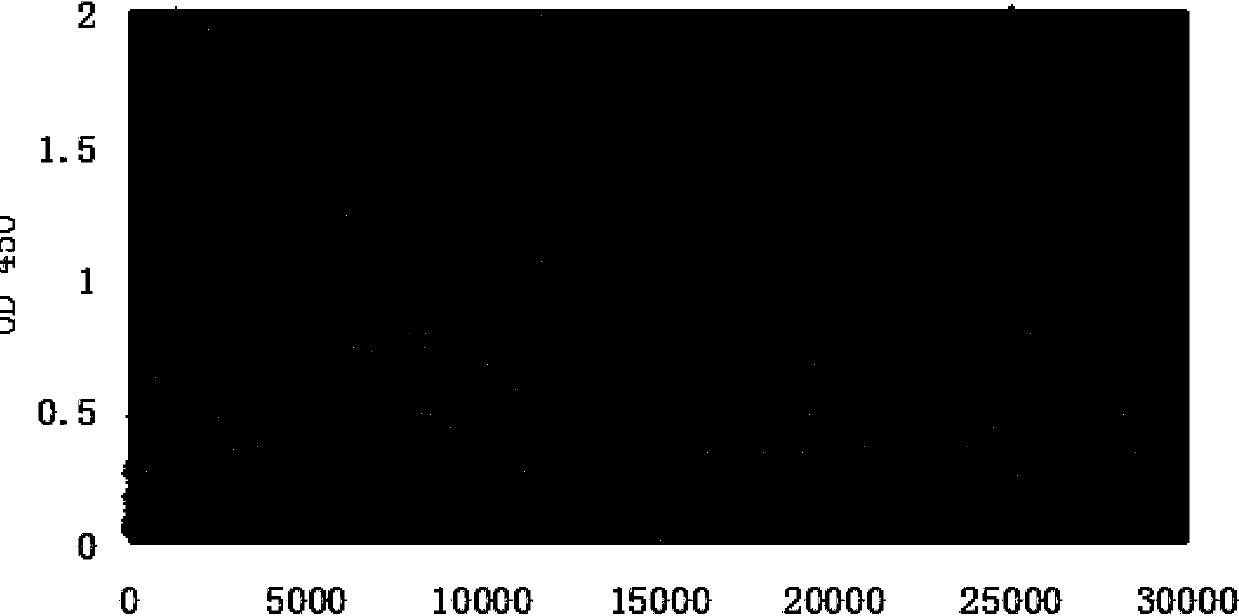
[0113] Take out at 225cm 2 The cell supernatant of the square flask cultured for about 10 days was centrifuged to remove cell debris, and the antibody was purified by rProteinA affinity chromatography. Cell culture supernatant at pH 7.4 was filtered through a 0.22 μm filter membrane and loaded directly, then washed with pH 7.4 PBS to 10 times the volume of the column bed, and then eluted with pH 3.0 glycine solution, collected and eluted in...
Embodiment 3
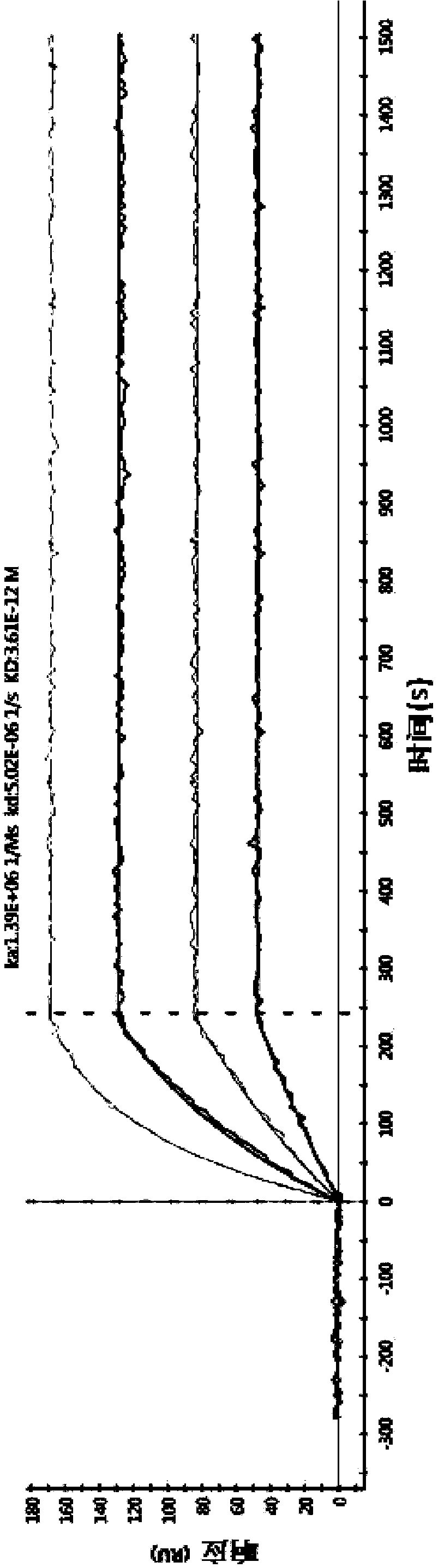
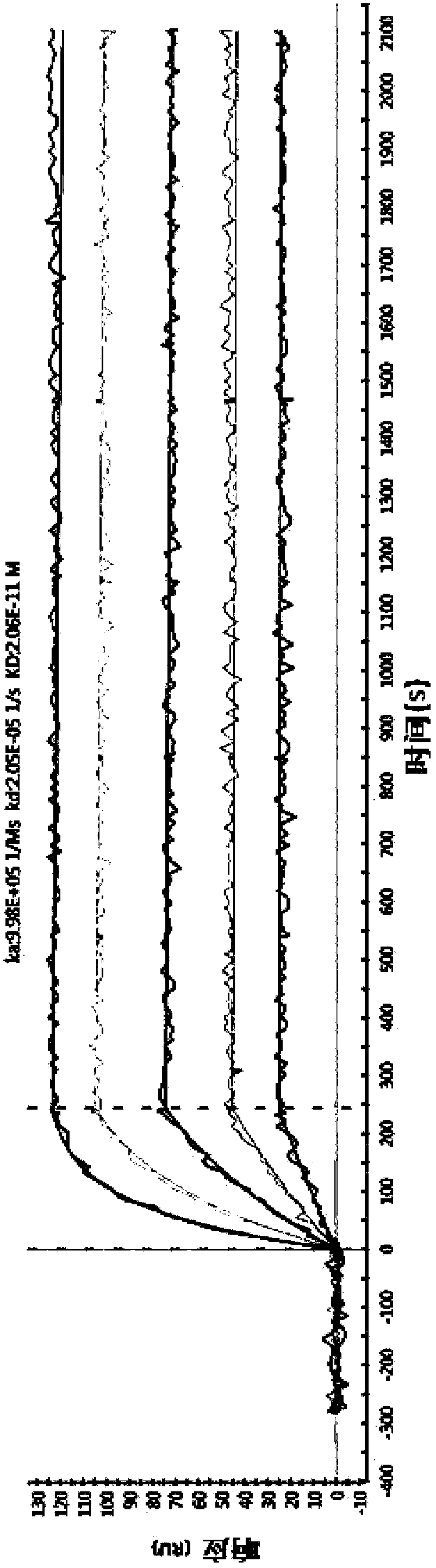
[0114] 3. Example 3: Affinity maturation
[0115] In order to improve the affinity of the antibody, the constructed single-chain antibody scFv was used as a template for random mutation, and the mutated single-chain antibody was subjected to phage display, and then screened by ELISA to obtain an antibody with higher affinity.
[0116] 3.1 Sequence the heavy and light chains of Anti-TNFα
[0117] After immunization, fusion, and monoclonalization, anti-TNFα monoclonal antibody cell lines were selected as templates for antibody affinity maturation experiments based on the results of affinity and cell function experiments.
[0118] Sequence analysis of heavy and light chains of antibody genes. The total RNA of the anti-TNFα monoclonal antibody cells was used by Invitrogen Reagent kit (15596-026) was used for extraction; then random primers in the 5'RACE FULL kit (D315) of Takara Company were used, and total RNA was used as a template to reverse transcribe into first-strand eDN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com