A new thioxanthone photoinitiator and its application in uv-led light curing
A UV-LED, photoinitiator technology, applied in the field of new thioxanthone photoinitiators, can solve problems such as poor compatibility, difficult purification, and difficult preservation, and achieve good compatibility, low extraction, and convenience. Effects of storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
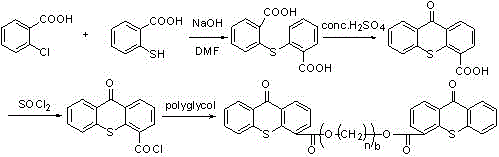
[0060] Example 1: Preparation of 2-(2'-carboxy-phenylthio)-benzoic acid
[0061] In a 250mL four-neck flask equipped with mechanical stirring, add 47.0g 2-chlorobenzoic acid, 46.2g 2-mercaptobenzoic acid, 100ml DMF, 13.2gNaOH, heat to reflux, reflux for 5 hours, cool down, distill out DMF, add 100mL of water, add 50mL of concentrated hydrochloric acid dropwise under stirring, adjust the pH value to 1, a large amount of solids precipitate out, filter, and add 150mL of methanol to recrystallize the obtained solids to obtain 74.0g of light yellow needle-shaped crystals, the yield is 90%, and the content is ≥98%. .
[0062] 1 H NMR (DMSO) δ: 7.10 (m, 2H), 7.38 (m, 2H), 7.46 (m, 2H), 7.84 (m, 2H), 13.12 (s, 2H).
Embodiment 2
[0063] Embodiment 2: the preparation of thioxanthone-4-carboxylic acid:
[0064] Add 40.0g of 2-(2’-carboxy-phenylthio)-benzoic acid, 3.0g of concentrated sulfuric acid (98%), and 30mL of xylene into a 250mL four-neck flask equipped with mechanical stirring, and heat to reflux for dehydration for 5 hours. Cool down, add 50mL of water, solid precipitates, and filter. The obtained solid was recrystallized by adding 30 mL of methanol to obtain 33.6 g of yellow powder with a yield of 90% and a content of ≥99%.
[0065] 1 H NMR (DMSO) δ: 7.10-7.80 (m, 6H), 8.40 (m, 1H), 12.81 (s, 1H).
Embodiment 3
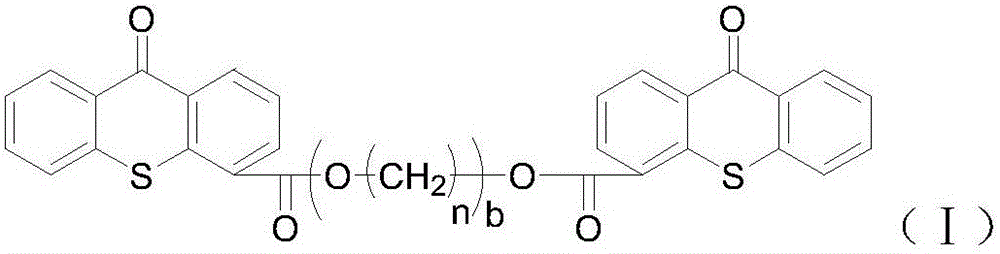
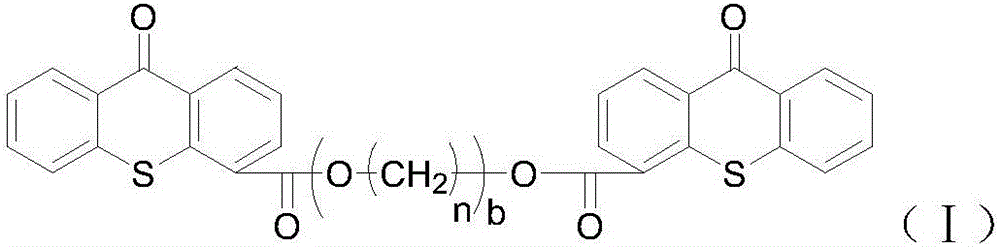
[0066] Embodiment 3: Preparation of thioxanthone-4-carboxylic acid polyethylene glycol-600-diester
[0067] Into a 250 mL four-necked flask, thioxanthone-4-carboxylic acid (51.20 g, 0.2 mol) and chlorobenzene (150 mL) were added in sequence. With mechanical stirring, thionyl chloride (50 mL) was slowly added dropwise at room temperature, and the temperature was raised to reflux. TLC monitored until the reaction of thioxanthone-4-carboxylic acid was complete. The unreacted thionyl chloride and chlorobenzene were distilled off under reduced pressure to obtain a crude yellow solid, which was washed with chlorobenzene to obtain a yellow solid for future use.
[0068] Into a 250 mL four-necked flask, the above solid crude product (27.40 g, 0.1 mol), chlorobenzene (100 mL), and triethylamine (10 mL) were sequentially added. With mechanical stirring, polyethylene glycol 600 (0.12 mol) was slowly added dropwise at room temperature, the reaction temperature was controlled at 40°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com