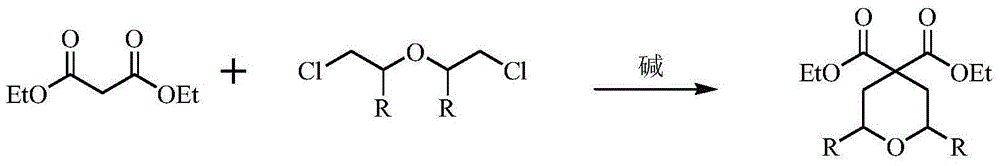
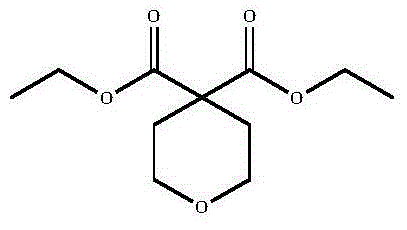
Method for preparing 4,4-pyran diethyl dicarboxylate derivative
A technology of diethylpyranedicarboxylate and diethylmalonate, which is applied in the field of synthesis of photoresist materials, can solve the problems of limited wide application, long reaction time, and low reaction yield, and achieve cheap and easy raw materials. High efficiency, short reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of 4,4-diethyl pyrandicarboxylate (sodium tert-amylate is the base)
[0020] Add 48.0g (0.3mol) of diethyl malonate, 42.6g (0.3mol) of dichloroethyl ether, 480g of dry DMF, and 0.6g of cuprous iodide to a 1L three-necked flask protected by nitrogen, and the obtained system is light yellow and transparent solution. The temperature was raised to 100°C with stirring. A DMF solution of sodium tert-amyloxide (prepared from 69.3g sodium tert-amyloxide and 277.2g DMF) was added dropwise. Refluxed for 2 hours, followed by GC until no raw material remained, evaporated the solvent under reduced pressure, and continued to distill under reduced pressure to obtain 61.5 g of colorless liquid, yield: 89.1%.
[0021] 1H NMR (CDCl 3 , TMS, 500MHz): 4.121-4.132 (q, 4H), 1.842-1.851 (m, 4H), 1.124-1.138 (t, 6H); GC-MS: 229 (M+).
[0022]
Embodiment 2
[0023] Example 2 Preparation of 4,4-diethyl pyrandicarboxylate (sodium tert-butoxide is the base)
[0024] Add 48.0g (0.3mol) of diethyl malonate, 42.6g (0.3mol) of dichloroethyl ether, 480g of dry DMF, and 0.6g of cuprous iodide to a 1L three-necked flask protected by nitrogen, and the obtained system is light yellow and transparent solution. The temperature was raised to 100°C with stirring. A DMF solution of 60.5 g of sodium tert-butoxide (prepared from 60.5 g of sodium tert-butoxide and 202 g of DMF) was added dropwise. Refluxed for 2 hours, followed by GC until there was no raw material left, the solvent was evaporated under reduced pressure, and the distillation was continued under reduced pressure to obtain 58.0 g of a colorless liquid, yield: 84.1%.
[0025] 1H NMR (CDCl 3 , TMS, 500MHz): 4.121-4.132 (q, 4H), 1.842-1.851 (m, 4H), 1.124-1.138 (t, 6H); GC-MS: 229 (M+).
[0026]
Embodiment 3
[0027] Example 3 Preparation of 4,4-diethyl pyrandicarboxylate (potassium tert-butoxide is the base)
[0028] Add 48.0g (0.3mol) of diethyl malonate, 42.6g (0.3mol) of dichloroethyl ether, 480g of dry DMF, and 0.6g of cuprous iodide to a 1L three-necked flask protected by nitrogen, and the obtained system is light yellow and transparent solution. The temperature was raised to 100°C with stirring. A DMF solution of 71.2 g of potassium tert-butoxide (prepared from 71.2 g of potassium tert-butoxide and 284.8 g of DMF) was added dropwise. Refluxed for 2 hours, followed by GC until no raw material was left, the solvent was evaporated under reduced pressure, and the distillation was continued under reduced pressure to obtain 59.5 g of colorless liquid, yield: 86.2%.
[0029] 1H NMR (CDCl 3 , TMS, 500MHz): 4.121-4.132 (q, 4H), 1.842-1.851 (m, 4H), 1.124-1.138 (t, 6H); GC-MS: 229 (M+).
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com