Application of artesunate and podophyllotoxin conjugates in anti-leukemia drugs and preparation method
A technology of podophyllotoxin and artesunate, which can be used in drug combination, antineoplastic drugs, organic chemistry, etc., and can solve the problems of high toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of artesunate-podophyllotoxin conjugates (1)
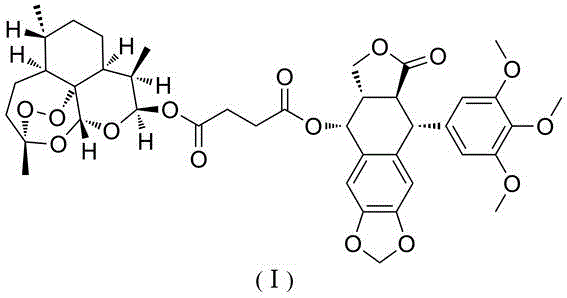
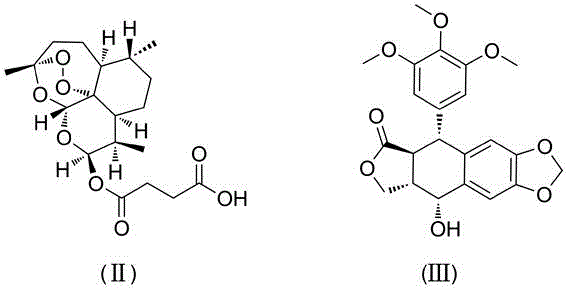
[0016] Add artesunate (0.25mmol), podophyllotoxin (0.25mmol) and 4-dimethylaminopyridine (0.3mmol) into a 10mL pear-shaped bottle, then add N,N-dimethylformamide (4mL) to dissolve , add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.5mmol) under ice-water bath cooling, stir and react at 25°C for 6 hours, pour the reaction solution into water, stir and filter with suction , washed with water, and dried in vacuum, the obtained crude product was subjected to column chromatography (the eluent was a mixed solution of ethyl acetate and petroleum ether at a volume ratio of 1:2) to obtain the coupled artesunate and podophyllotoxin shown in formula (I). material with a yield of 60%.
[0017] MP: 139-141°C; IR (KBr) 3622, 2938, 1779, 1588, 11485, 1456, 1420, 1376, 1331, 1240, 1127, 1037, 927 cm -1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 6.80 (s, 1H), 6.52 (s, 1H), 6.39 (s, 2H), 5.98 (dd, J = 5.5, 1.3 H...
Embodiment 2
[0018] Example 2: Preparation of artesunate-podophyllotoxin conjugates (2)
[0019] Add artesunate (0.25mmol), podophyllotoxin (0.5mmol) and 4-dimethylaminopyridine (0.3mmol) into a 10mL pear-shaped bottle, then add dichloromethane (4mL) to dissolve, and add it under cooling in an ice-water bath 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.5mmol), stirred and reacted at 30°C for 4 hours, poured the reaction liquid into water, stirred, filtered with suction, washed with water, and dried in vacuum. The resulting crude product was subjected to column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether at a volume ratio of 1:2) to obtain the conjugate of artesunate and podophyllotoxin represented by formula (I), with a yield of 50 %.
Embodiment 3
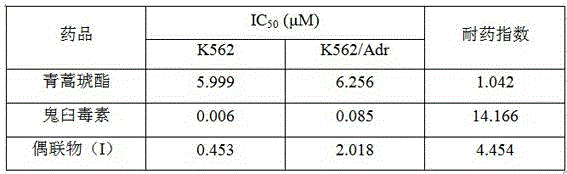
[0020] Example 3: Proliferation inhibitory activity of conjugates represented by formula (I) on human leukemia cells
[0021] CCK-8 staining method:
[0022] Cells were seeded in 96-well plates in CO 2 After culturing in the incubator for 24 hours, add drug-containing medium with a concentration gradient, set up a negative control group, a positive control group and a vehicle group at the same time, take out the culture plate after continuing to cultivate for 72 hours, add 10 microliters of CCK-8 solution, and cultivate for 3 After 1 hour, select 450nm on the microplate reader to measure the absorbance value (OD value), and calculate the inhibition rate of each group, and the inhibition rate (%) = [(OD value of the negative control group-OD value of the experimental group) / OD of the negative control group Value] × 100%. Using SPSS17.0 software to calculate the half inhibitory concentration (IC 50 ).
[0023] In this experiment, according to the CCK-8 method, artesunate and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com