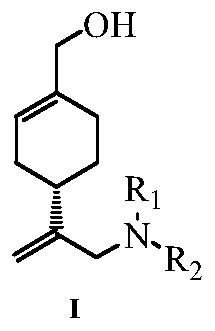
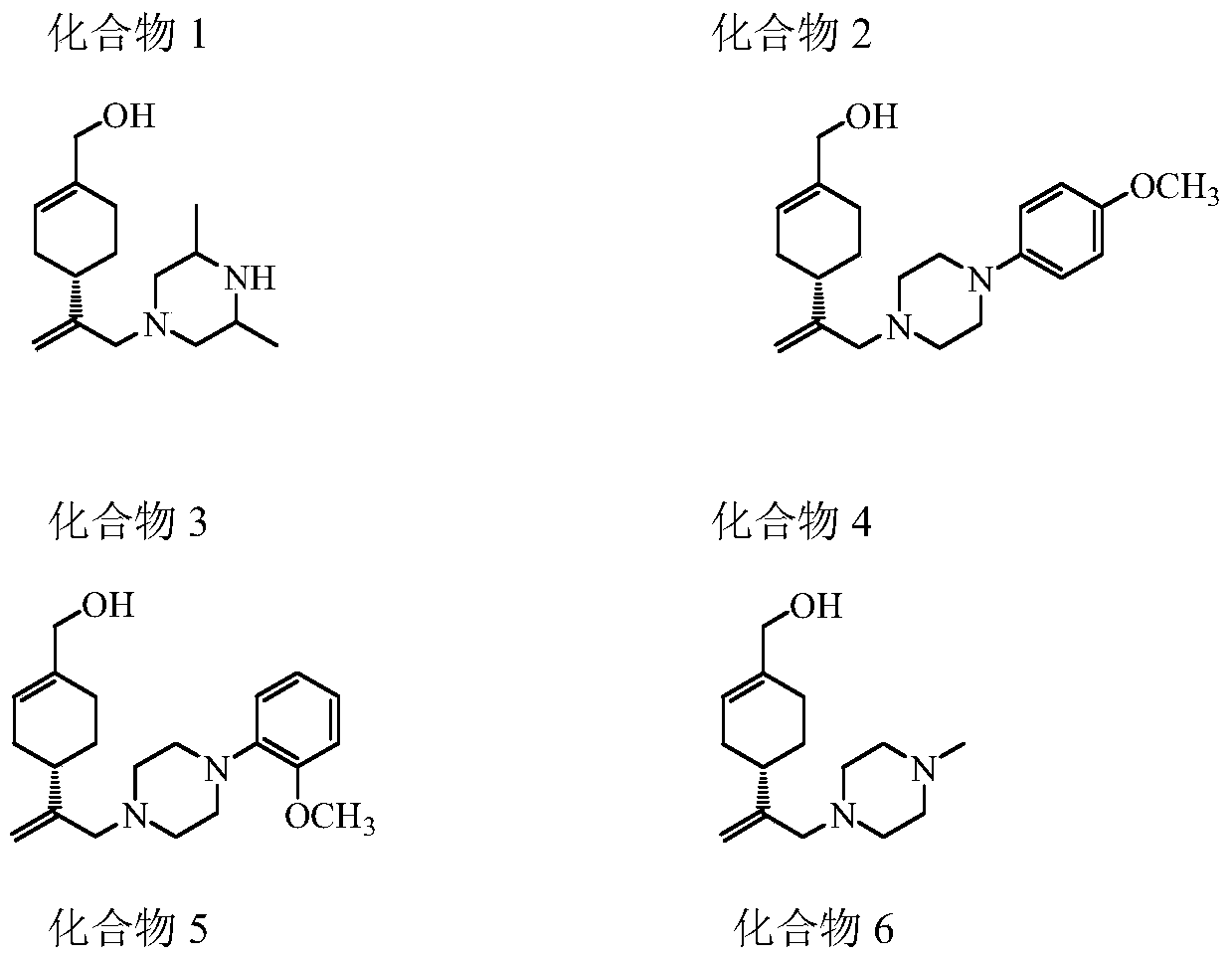
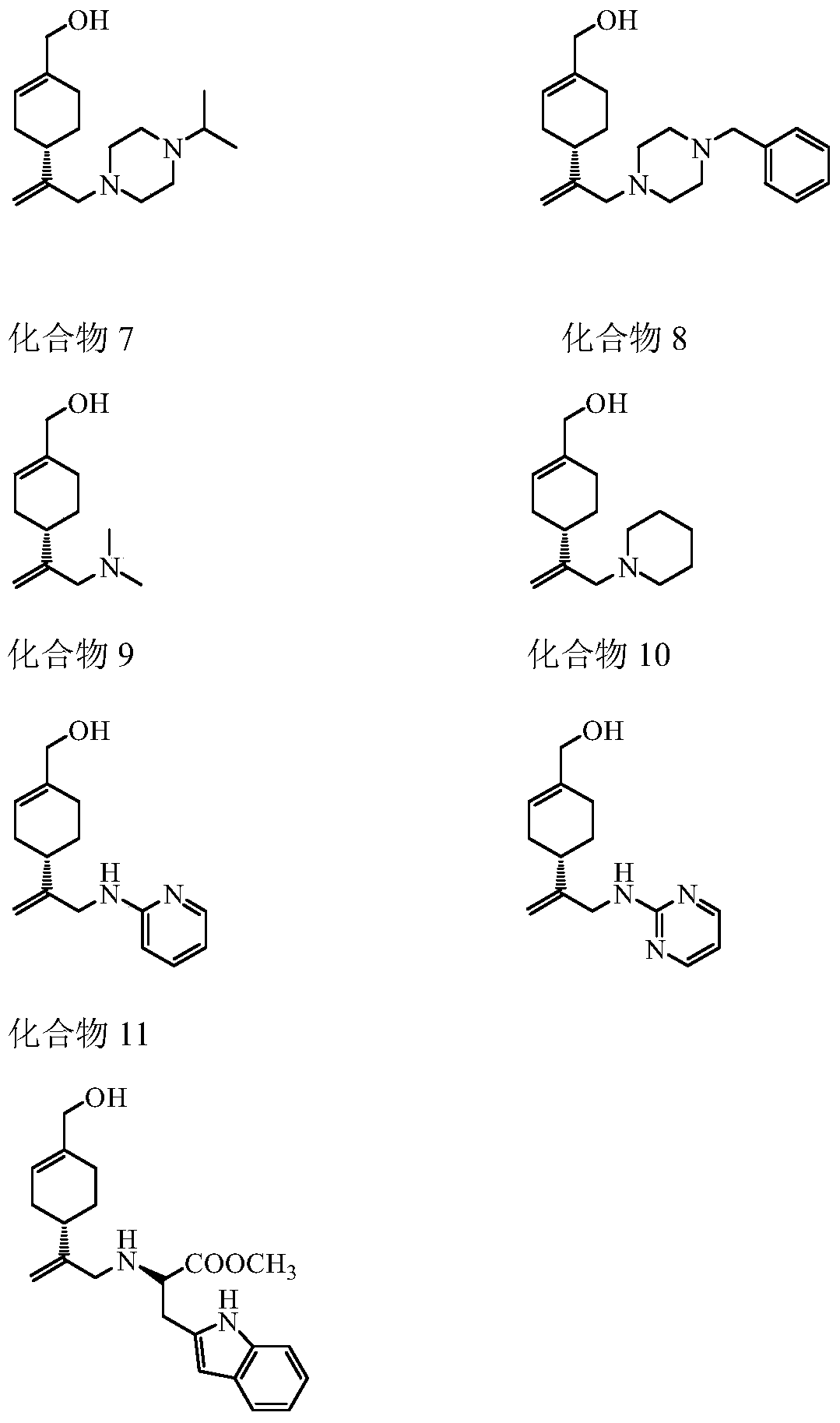
Perillyl alcohol derivatives and their preparation and application
A derivative, perillyl alcohol technology, applied in the field of perillyl alcohol derivatives, can solve the problems of low bioavailability of oral administration, poor water solubility of perillyl alcohol, limited clinical application and the like, and achieves a simple and feasible preparation method, good resistance Cancer active, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 perillyl alcohol Synthesis
[0026] Dissolve 0.1 mol perillaldehyde in 100 mL of absolute ethanol, add 0.2 mol NaBH in batches under ice-cooling 4 , After the addition was completed, the reaction was carried out at room temperature for 3h. Distill ethanol off under reduced pressure, add 30mL water and 30mL dichloromethane, transfer to a separatory funnel, separate the organic phase, extract the water phase with dichloromethane, combine the organic phases, wash with water and saturated brine, and dry over anhydrous sodium sulfate . After concentration, perillyl alcohol was obtained as a colorless transparent liquid with a yield of 86.8%.
Embodiment 2
[0027] Example 2 Synthesis
[0028] Add 25 mL of acetic anhydride dropwise to a solution of 0.08 mol of the product of Example 1 in 25 mL of pyridine, and react at room temperature for 4 h. Add 2 mL of methanol and 50 mL of ethyl acetate, transfer to a separatory funnel, separate the organic phase, wash with saturated sodium bicarbonate and saturated brine, and dry over anhydrous sodium sulfate. Concentrate to obtain a colorless transparent liquid with a yield of 95.4%.
Embodiment 3
[0029] Example 3 Synthesis
[0030] Dissolve 0.06mol of the product of Example 2 and 0.09mol of glacial acetic acid in 150mL of dichloromethane, and slowly add 0.24mol of sodium hypochlorite aqueous solution (containing 10% available chlorine) dropwise under ice cooling. After the addition, the reaction was continued for 30 minutes. Add 50mLNa 2 SO 3 aqueous solution, the organic layer was separated, the aqueous layer was extracted with dichloromethane, the organic phases were combined, washed with water and saturated NaCl solution, and dried over anhydrous sodium sulfate. Concentrate to obtain a light yellow liquid with a yield of 85.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com