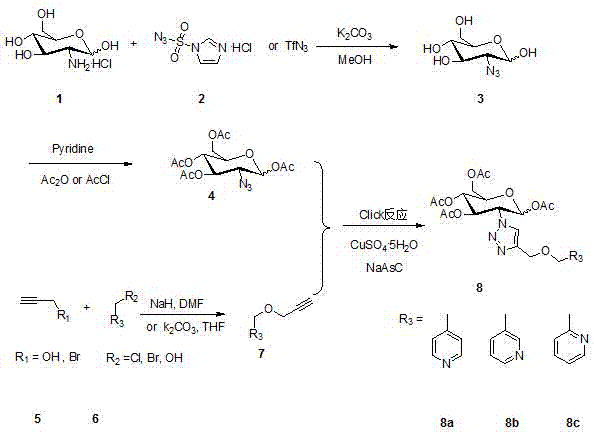
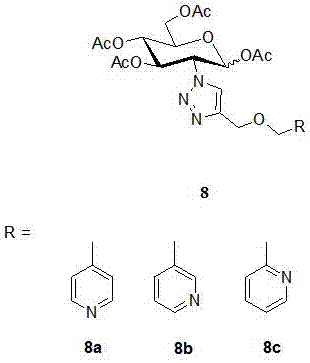
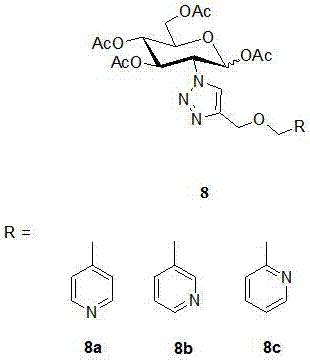
2-(1',2',3'-triazole-4'-oxymethylenepyridine)-1,3,4,6-o-acetyl-d-glucose and its preparation method and application
A technology of oxymethylenepyridine and glucose, applied in 2‑(1',2',3'‑triazole‑4'‑oxymethylenepyridine)‑1,3,4,6‑O‑ Acetyl-D-glucose and its preparation and application fields can solve problems such as bone marrow suppression, gastrointestinal dysfunction, and lower quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 2-azido-1,3,4,6-O-acetyl-D-glucose intermediate, methanol as solvent, azide reagent as trifluoromethanesulfonyl azide.
[0026] Weigh compound 1 (4.313 g, 20 mmol) dissolved in triethylamine (7.5 mL, 54 mmol) and CuSO 4 .5H 2 O (50 mg, 0.2 mmol) in water (84 mL) was filled with nitrogen for protection, and then stirred continuously for 30 min under ice-water bath conditions. Compound 2 (5.030 g, 24 mmol) was added under constant stirring, and the reaction was continued for half an hour, then the ice-water bath was removed, and the system was allowed to react at room temperature for 24 hours.
[0027] TLC detection reaction. The solvent was evaporated under reduced pressure. The residue was azeotroped with 50 mL of toluene to remove water. After adding pyridine (100 mL, 20 mmol) to the above residue, Ac 2 O (15 mL, 160 mmol), stirred overnight. The solvent was distilled off under reduced pressure, and 50 mL of water was added to the residue....
Embodiment 2
[0028] Example 2: Synthesis of 4-pyridyl propargyl ether 7a, using tetrahydrofuran as solvent.
[0029] Weigh 4-pyridinemethanol (303 mg, 2.774 mmol) and dissolve it in dry tetrahydrofuran (2 mL). Then use nitrogen protection, ice water external bath, and stir for 30 minutes. Weigh NaH (133 mg, 3.329 mmol), add to the solution, and react for 30 minutes. Then 3-bromopropyne (413 mg, 3.472 mmol) was added. After continuing the reaction for 3 hours, 1 mL of water was added to quench the reaction in an ice bath. Extract with 100 mL of dichloromethane three times. Using TLC detection, the developer (PE: EA=4: 1) confirmed that the reaction was complete. Use silica gel column for purification, eluent (PE:EA=2:1). The product was subjected to vacuum distillation to remove the solvent, and finally the product 7a (151 mg, yield 37%) was obtained.
[0030] Example 3 Synthesis of 3-pyridine propargyl ether 7b, N, N'-dimethylformamide as solvent.
[0031] Weigh 3-pyridinemethanol (...
Embodiment 4
[0032] Example 4: Synthesis of 2-pyridine propargyl ether 7c, using acetonitrile as solvent.
[0033]Weigh 2-pyridinemethanol (300 mg, 2.74 mmol) and dissolve it in dry acetonitrile (5 mL). Then use nitrogen protection, ice water external bath, and stir for 30 minutes. Potassium carbonate (460 mg, 6.9 mmol) was weighed, added to the solution, and reacted for 30 minutes. Then 3-bromopropyne (413 mg, 3.472 mmol) was added. After continuing the reaction for 3 hours, 1 mL of water was added to quench the reaction in an ice bath. Extract with 100 mL of dichloromethane three times. Using TLC detection, the developer (PE: EA = 4: 1) confirmed that the reaction was complete. Use a silica gel column for purification, eluent (PE: EA = 2: 1). The product was distilled under reduced pressure to remove the solvent, and finally the product 2-pyridine propargyl ether 7c (320 mg, yield 79%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com