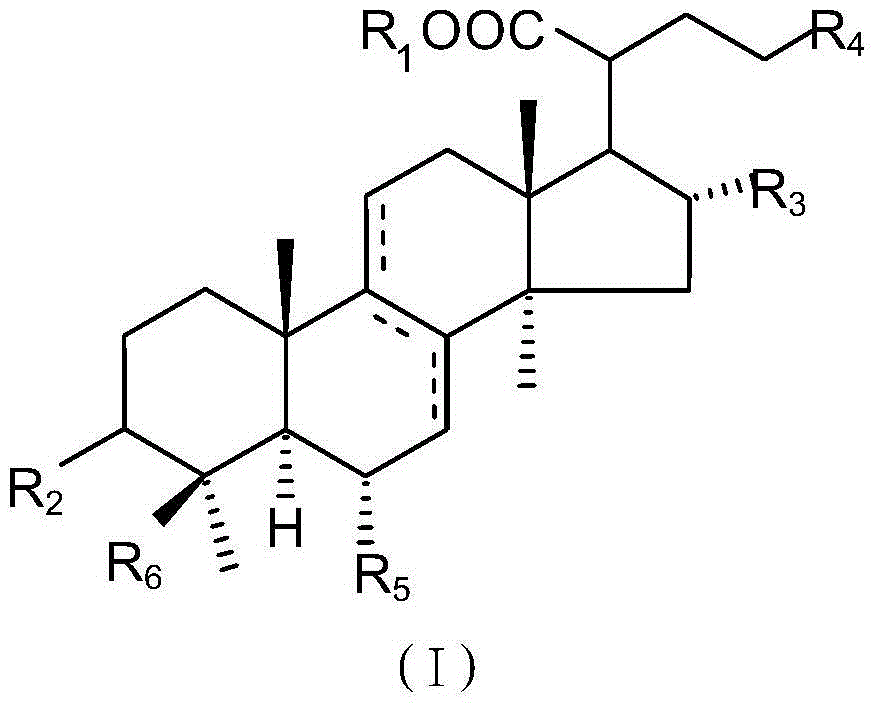
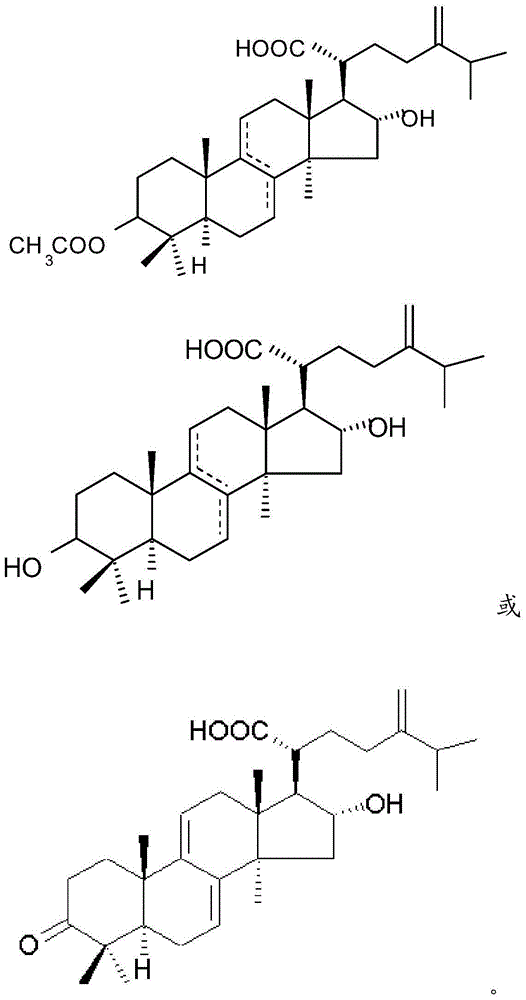
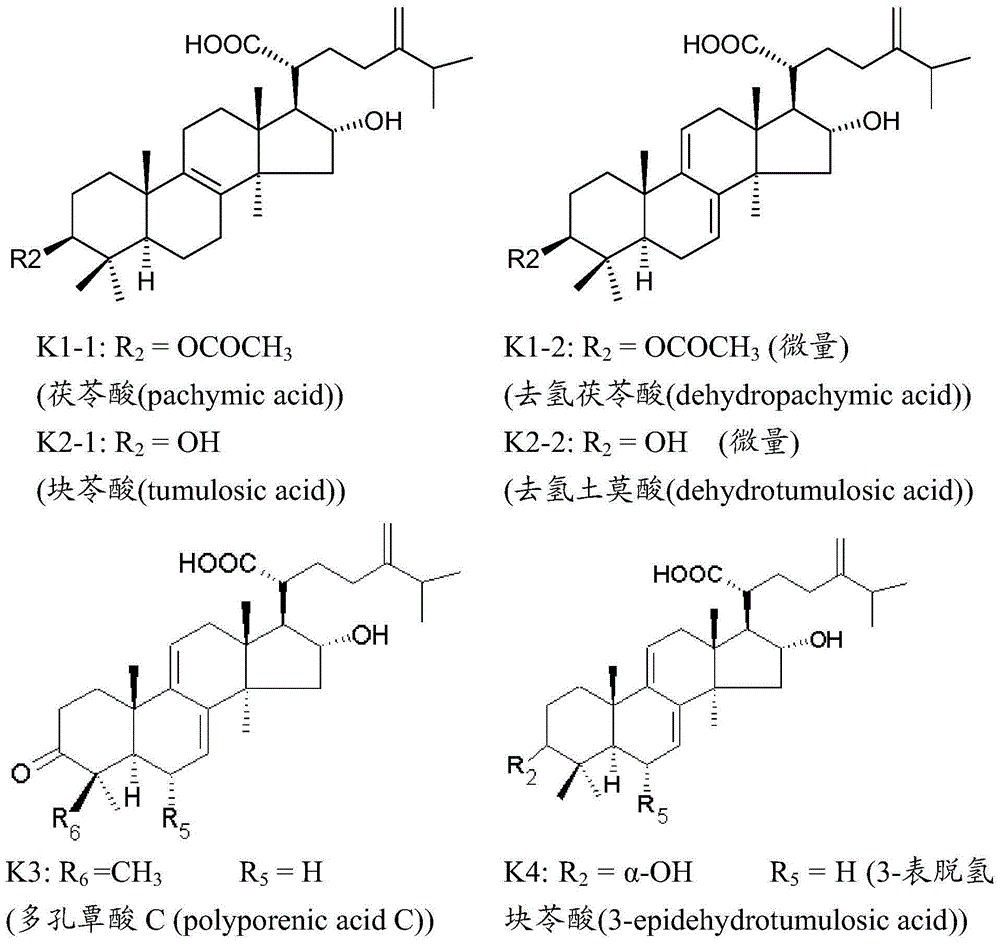
Medicine composition for treating disease caused by immunity imbalance and poria cocos extract
A technology for immune disorders and medicines is applied in the field of pharmaceutical compositions and Poria cocos extracts for the treatment of diseases caused by immune disorders, and can solve the problems of undiscovered lanostane and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Take 30 kg of Poria cocos produced in Yunnan, grind it into powder, extract with 120 L of alcohol (concentration 95%) for 24 hours, and separate by filtration. The aforementioned extraction and solid-liquid separation were repeated three times. The filtrates were combined and concentrated to obtain 265.2 g of dry extract. The dry extract was then extracted with a two-phase extractant (hexane: 95% methanol = 1:1). The methanol layer was taken out and pre-concentrated to obtain 246.9 g of dry solid. The dry solid is separated by silica gel column chromatography, and the silica gel column is filled with silica gel 10-40 times the weight of the dry solid, purchased from Merck Company, Silica gel 60, 70-230 mesh. Using dichloromethane / methanol mixture as eluent, elution was carried out at the ratio of 96:4, 90:10, and 0:100 in sequence, and the eluate was subjected to silica gel thin-layer chromatography Method (Thin Layer Chromatography) (ultraviolet light and iodine for...
Embodiment 2
[0052] After boiling 100 kg of Poria cocos with 800 kg of water for 3 hours, let it stand and cool to 50°C, adjust the solution to pH 11 with 5N NaOH, and then stir the solution for 3 hours. Then use a centrifuge to separate the liquid and solid, then add the solid with 800 kg of water, adjust the pH to 11 with NaOH, stir and separate with a centrifuge, and remove the solid. The two liquids were combined, and the liquid was concentrated in vacuo at 50°C to 100 kg of solution, and then 3N HCl was added to pH 6.5, resulting in a precipitate. Separate the precipitate, and then with 40L H 2 O was washed, and then the precipitate was separated with a centrifuge, and 8 L of water was added to spray dry (spray dry) to obtain about 380 g of powder. Then extract the powder three times with 4L of alcohol, combine the extracts and concentrate to obtain 238.9 grams of alcohol extract (PCE), and then separate the extract by HPLC. The main components of each gram of the extract are K2185.9...
Embodiment 3
[0054] Experiment of Treating Asthmatic Mice with Poria Cocos Extract and Its Purified Lanostane Compounds
[0055] In this example, after the mice were sensitized with ovalbumin (OVA) as an allergen, the presence of OVA-specific IgE antibodies in the mice confirmed that the animals had been induced with symptoms of asthma. During the experiment, each group was fed with Poria cocos extract and its purified lanostane compounds, and one month later, the airway hyperresponsiveness of the lungs was tested on the asthmatic mice as an important indicator for determining the degree of asthma in the mice . This embodiment also detects various immune cells in the bronchoalveolar lavage fluid of mice to observe whether various immune cells are affected, especially whether neutrophils and eosinophils are affected. In this embodiment, chemokine (Eotaxin) is also used as an important observation factor to observe whether there is a major change in the secretion of cytokines.
[0056] exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com