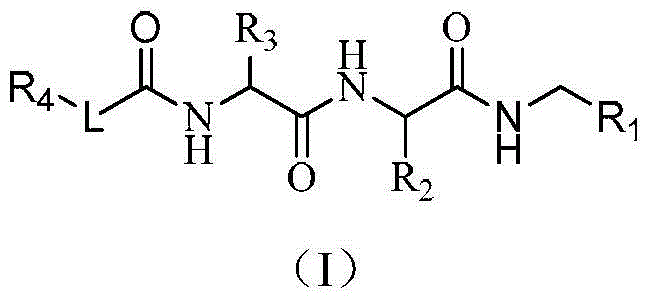
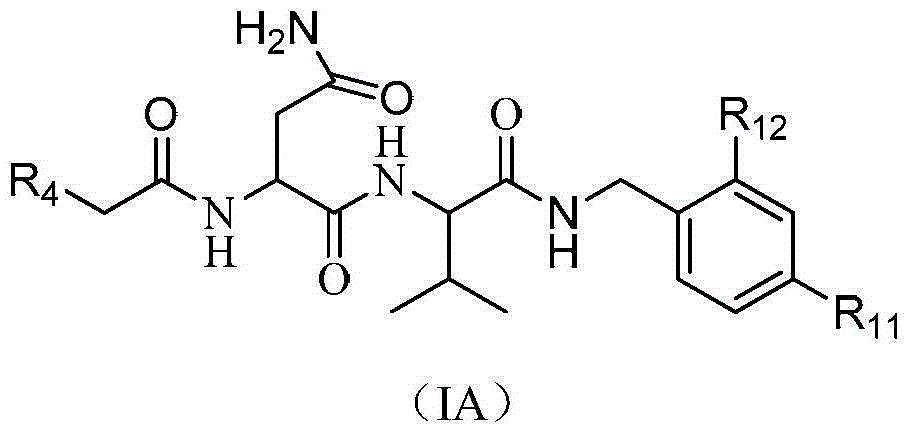
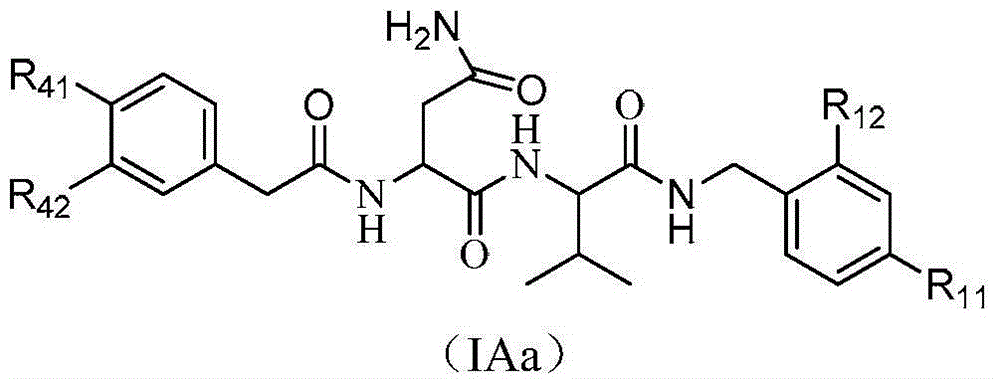
Dipeptide and tripeptide proteasome inhibitors as well as preparation method and pharmaceutical application thereof
A compound and stereoisomer technology, applied in the fields of dipeptide and tripeptide proteasome inhibitors and their preparation methods and pharmaceutical applications, can solve toxic and side effects, non-covalent proteasome inhibitors have less research, Insufficient drug safety and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Example 1: WK-3-18
[0167]
[0168] a)
[0169] Dissolve 2.1 g of white solid Boc-Asn-Val-OH (6.3 mmol) in 50 mL of DMF, add 1.2 g of 2-hydroxy-4-methoxybenzylamine hydrochloride (6.3 mmol), 2.4 g of HATU (6.3 mmol), respectively, 2.0 g DIEA (15.75 mmol). Stir overnight at room temperature, TLC detection, the reaction is complete, evaporate the solvent under reduced pressure, dissolve with ethyl acetate, wash with 1mol / L hydrochloric acid solution, saturated sodium carbonate solution, saturated sodium chloride solution, and the remaining organic layer is washed with After drying over sodium sulfate, the solvent was evaporated under reduced pressure, washed with a small amount of ethyl acetate, and filtered to obtain 2.5 g of a white solid with a yield of 85%.
[0170] b)
[0171] Dissolve the white solid (0.57 mmol) obtained in 266 mg1a in 8 mL of dichloromethane, add 2 mL of trifluoroacetic acid, stir at room temperature for 2 hours, TLC detects that the reactio...
Embodiment 2
[0172] Example 2: WK-3-21
[0173]
[0174] Dissolve the white solid (0.43 mmol) obtained in 200 mg1a in 8 mL of dichloromethane, add 2 mL of trifluoroacetic acid, stir at room temperature for 2 hours, TLC detects that the reaction is complete, evaporate the solvent under reduced pressure to obtain a white solid, dissolve it in In 20 mL of DMF, 75 mg of 3-indoleacetic acid (0.43 mmol), 164 mg of HATU (0.43 mmol), and 139 mg of DIEA (0.57 mmol) were added, respectively. Stir at room temperature overnight, TLC detection, the reaction is complete, the solvent is evaporated under reduced pressure, and after being partially dissolved with ethyl acetate, the solution is washed with 1mol / L hydrochloric acid solution, saturated sodium carbonate solution, saturated sodium chloride solution, distilled water, and evaporated under reduced pressure. Dry, wash with ethyl acetate, and filter to obtain 142 mg of a light red solid with a yield of 63%. mp:214℃-215℃. 1 H-NMR (CD 3 OD)δ7.48...
Embodiment 3
[0175] Example 3: WK-3-24
[0176]
[0177] Dissolve the white solid (0.43 mmol) obtained in 200 mg1a in 8 mL of dichloromethane, add 2 mL of trifluoroacetic acid, stir at room temperature for 2 hours, TLC detects that the reaction is complete, evaporate the solvent under reduced pressure to obtain a white solid, dissolve it in 91 mg of 4-felbinac (0.43 mmol), 164 mg of HATU (0.43 mmol), and 139 mg of DIEA (0.57 mmol) were added to 20 mL of DMF, respectively. Stir at room temperature overnight, TLC detection, the reaction is complete, the solvent is evaporated under reduced pressure, and after being partially dissolved with ethyl acetate, the solution is washed with 1mol / L hydrochloric acid solution, saturated sodium carbonate solution, saturated sodium chloride solution, distilled water, and evaporated under reduced pressure. Dry, wash with ethyl acetate, filter and recrystallize from methanol to obtain 105 mg of white solid with a yield of 43.6%. mp:231℃-232℃. 1 H-NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com