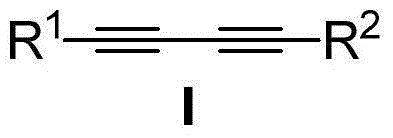Synthesis method for asymmetric conjugate diyne compound
A synthesis method and compound technology, applied in the field of organic synthesis, can solve problems such as the lack of breakthrough in asymmetric conjugated diyne compounds, precious metals, and narrow application range of substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0024] Synthesis of 2-methyl-6-phenylhexa-3,5-diyn-2-ol
[0025] A mixed solvent consisting of 0.01mmol copper powder, 0.04mmol TMEDA, 0.2mmol 3-methylbutynol-3, 0.28mmol phenylacetylene, 0.4mL chloroform and 1,4-dioxane was added to the reactor (volume ratio is [3:1]). Heat to 50°C, continue to stir for 12 hours, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, extract with dichloromethane, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product. rate of 81%. 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.48-7.46(m,2H),7.35-7.30(m,3H),2.45(s,1H),1.58(s,6H).
Synthetic example 2
[0027] Synthesis of 2-methyl-6-(naphthalene-1-yl)hexa-3,5-diyn-2-ol
[0028] A mixed solvent composed of 0.02mmol copper powder, 0.04mmol TMEDA, 0.2mmol 3-methylbutynol-3, 0.28mmol 1-acetylene naphthalene, 0.4mL chloroform and 1,4-dioxane was added to the reactor ( The volume ratio is [3:1]). Heat to 50°C, continue to stir for 12 hours, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, extract with dichloromethane, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product. rate of 72%. 1 H NMR (400MHz, CDCl 3 ,TMS): δ8.32(d,J=8.3Hz,1H),7.85(d,J=8.2Hz,2H),7.74(dd,J=7.2,0.7Hz,1H),7.60-7.50(m, 2H),7.43-7.39(m,1H),2.23(s,1H),1.63(s,6H).
Synthetic example 3
[0030] Synthesis of 2-methyl-6-(p-tolyl)hexa-3,5-diyn-2-ol
[0031]Add 0.01mmol copper powder, 0.02mmol TMEDA, 0.2mmol 3-methylbutynol-3, 0.28mmol 4-ethynyltoluene, 0.4mL chloroform and 1,4-dioxane to the reactor (The volume ratio is [3:1]). Heat to 50°C, continue to stir for 12 hours, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, extract with dichloromethane, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product. rate of 72%. 1 H NMR (400MHz, CDCl 3 ,TMS): δ7.38(d,J=8.1Hz,2H),7.12(d,J=8.1Hz,2H),2.34(s,3H),2.28(s,1H),1.57(s,6H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com