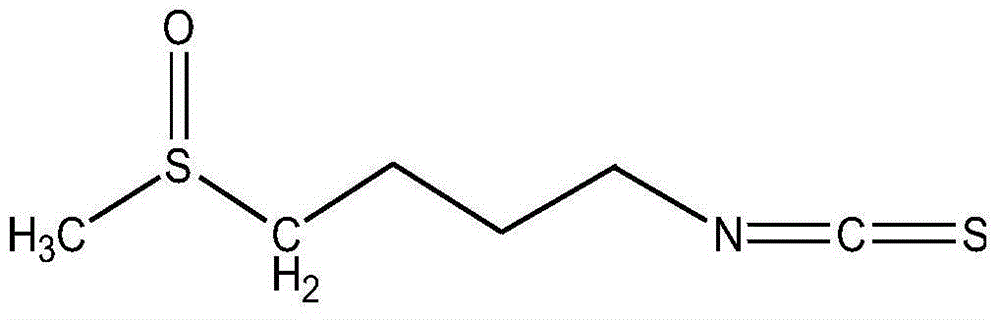
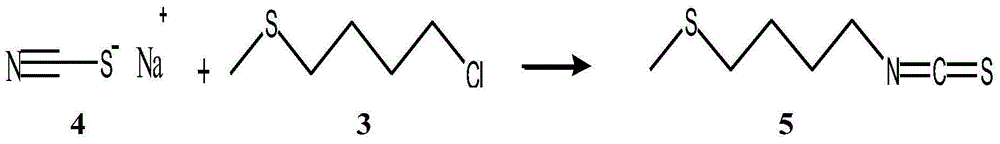Chemical synthetic method for raphanin
A substrate, ZIF-9 technology, applied in the direction of organic chemistry, etc., can solve the problem of many steps, and achieve the effect of less steps, convenient operation and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
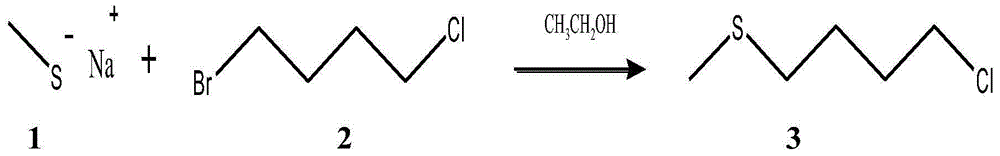
[0022] Embodiment 1: (1) synthetic 1-chloro-4-methylthiobutane (compound 3)
[0023] Place the 250mL round-bottom flask reactor in a fume hood, weigh 1.76g (25mmol) sodium methyl mercaptide into the reactor, add 50mL ethanol to dissolve, and then place the round-bottom flask reactor in an ice-water bath at 0°C , quickly inject 4.29g (25mmol) 1-bromo-4-chlorobutane, stir the reaction for 20min under the reaction condition of 0°C, observe the formation of NaBr precipitate, then raise the reaction temperature to room temperature, continue at room temperature The reaction was stirred overnight, and then the reaction mixture was filtered and the solvent was removed to obtain 3.35 g of an oily product (Compound 3), with a yield of 96.5%. Compound 3 was then stored at low temperature.
[0024] Or adopt the following method to synthesize 1-chloro-4-methylthiobutane (compound 3)
[0025] Place the 250mL round-bottom flask reactor in a fume hood, weigh 1.76g (25mmol) sodium methyl mer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com