Gemcitabine chemical transfer prodrug, preparation method and applications thereof
A technology of chemical delivery and gemcitabine, applied in chemical instruments and methods, organic chemistry, pharmaceutical formulations, etc., can solve the problems of limiting drug therapeutic effect, limited ability, and inability to fully meet clinical treatment, and achieve the effect of increasing drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The preferred embodiments of the present invention are given below in conjunction with the accompanying drawings to describe the technical solution of the present invention in detail.
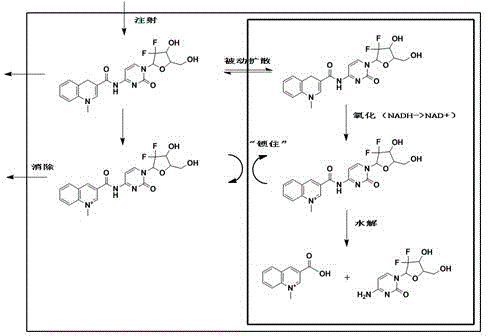
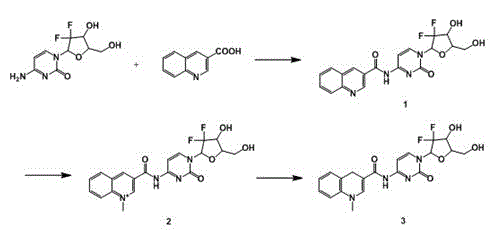
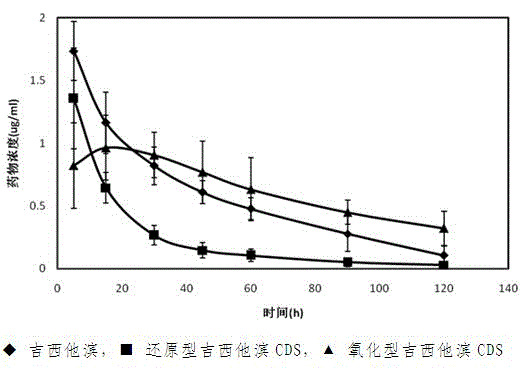
[0027] Mechanism of drug operation of the present invention such as figure 1 As shown, the gemcitabine chemical delivery prodrug is based on the oxidation-reduction reaction of dihydropyridine-pyridinium ions, and the drug gemcitabine is connected with the targeting agent 3-quinolinic acid to form a CDS conjugate (reduced gemcitabine chemical delivery prodrug), the high lipid solubility of the conjugate promotes its diffusion into the brain tissue, where the pyridine moiety in the CDS conjugate is absorbed by the coenzyme system (such as NAD(P)H←→NAD(P) + Coenzyme system) is oxidized to a water-soluble onium salt (oxidized gemcitabine chemical delivery prodrug), so that it cannot pass through the BBB and stay in the central nervous system, and release the drug slowly and continuously th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com