Carbazole group-containing azafluorene orange-light ionic-type iridium (III) complexes, and preparation method and applications thereof
A technology based on azafluorene and carbazole, which is applied in the field of organic electroluminescence display and can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
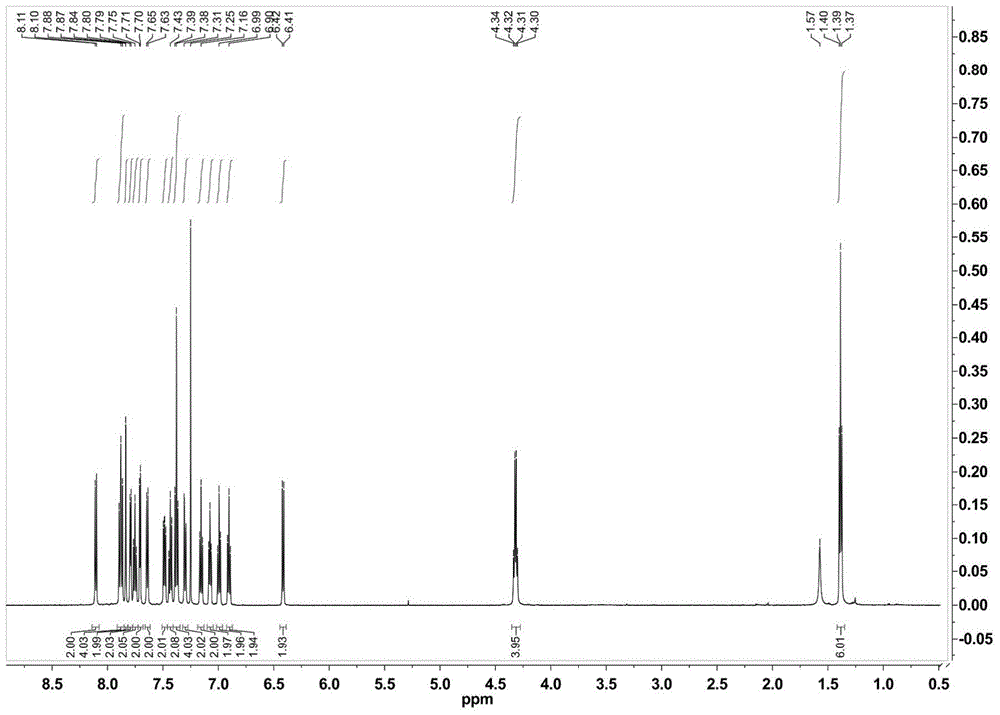
[0033] Synthesis of complex Ir1:
[0034] The auxiliary ligand 9,9-bis(9-ethylcarbazol-3-yl)-4,5-diazafluorene (ECAF) (0.42 g) containing 0.76 mmol of bicarrier groups was mixed with 0.33 mmol[Ir(ppy) 2 Cl] 2 (0.35 g) of the mixture was dissolved in 30 ml of ethylene glycol monoethyl ether, and reacted at 130° C. for 10 hours under the protection of nitrogen. Then cool to room temperature, add 20mL of deionized aqueous solution that is dissolved with 0.87g ammonium hexafluorophosphate to the above-mentioned solution under stirring, produce a large amount of precipitation, suction filtration, the precipitation is passed through the column chromatography column (eluent is dichloromethane / Acetone=10:1) was purified to obtain the corresponding complex [Ir(ppy) 2 (ECAF)] PF 6 (Ir1).
Embodiment 2
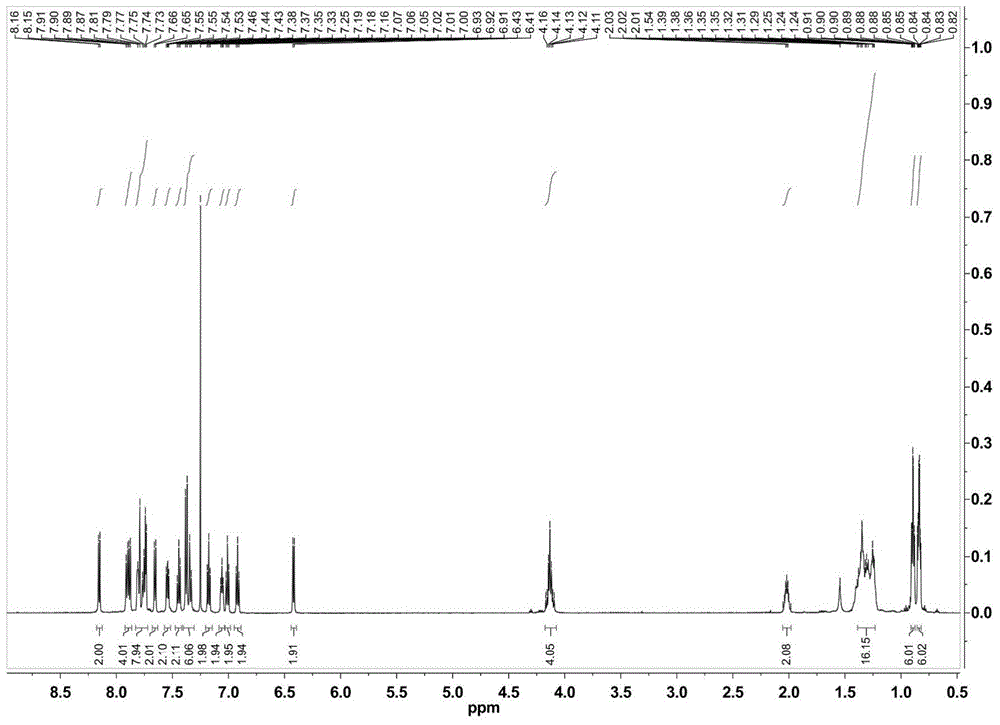
[0036] Synthesis of complex Ir2:
[0037] The auxiliary ligand 9,9-bis(9-ethylhexylcarbazol-3-yl)-4,5-diazafluorene (EHCAF) (0.49g) containing 0.76mmol of bicarrier groups was mixed with 0.33mmol [Ir(ppy) 2 Cl] 2 (0.35g) of the mixture was dissolved in 30ml of ethylene glycol monoethyl ether, and reacted at 150°C for 12 hours under the protection of nitrogen. Then cool to room temperature, add 20mL of deionized aqueous solution that is dissolved with 0.87g ammonium hexafluorophosphate to the above-mentioned solution under stirring, produce a large amount of precipitation, suction filtration, the precipitation is passed through the column chromatography column (eluent is dichloromethane / Acetone=10:1) was purified to obtain the corresponding complex [Ir(ppy) 2 (EHCAF)]PF 6 (Ir2).
Embodiment 3
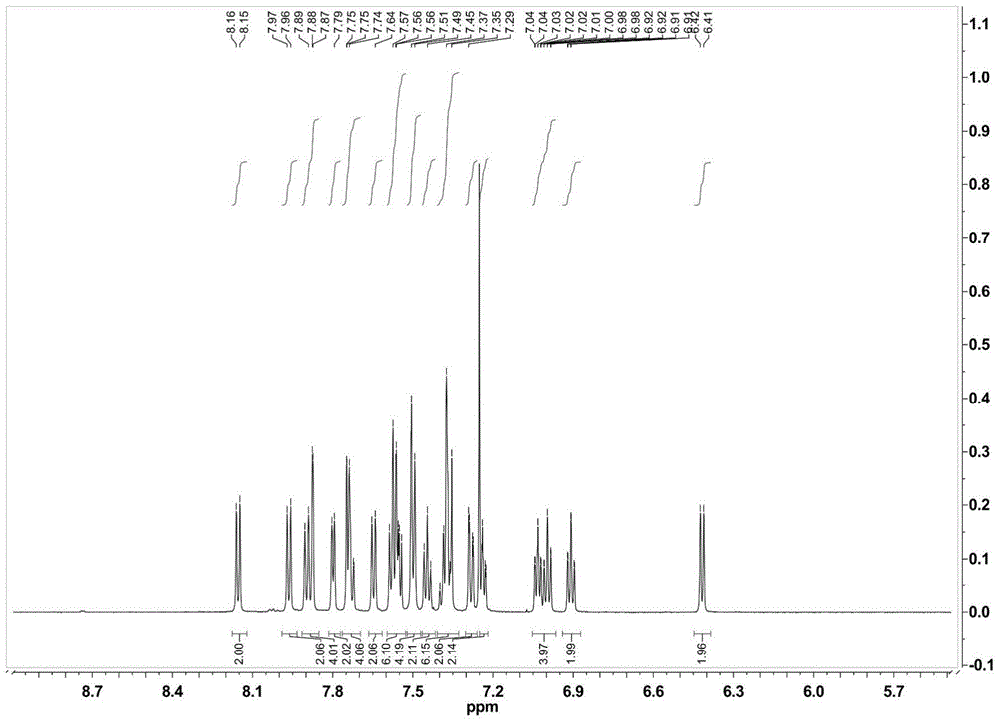
[0039] Synthesis of complex Ir3:
[0040] The auxiliary ligand 9,9-bis(9-phenylcarbazol-3-yl)-4,5-diazafluorene (PCAF) (0.49 g) containing 0.76 mmol of bicarrier groups was mixed with 0.33 mmol[Ir(ppy) 2 Cl] 2 (0.35 g) of the mixture was dissolved in 30 ml of ethylene glycol monoethyl ether, and reacted at 160° C. for 15 hours under the protection of nitrogen. Then cool to room temperature, add 20mL of deionized aqueous solution that is dissolved with 0.87g ammonium hexafluorophosphate to the above-mentioned solution under stirring, produce a large amount of precipitation, suction filtration, the precipitation is passed through the column chromatography column (eluent is dichloromethane / Acetone=10:1) was purified to obtain the corresponding complex [Ir(ppy) 2 (PCAF)]PF 6 (Ir3).
[0041] In the above-mentioned embodiment, the deionized aqueous solution of ammonium hexafluorophosphate can be replaced by an aqueous solution of ammonium tetrafluoroborate, and the chemical fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com