Application of compound T521 or analogues thereof in preparation of antitumor drug
A technology of similar structures and compounds, which is applied in the application field of compound T521 or its structural analogs to prepare anti-tumor drugs, and can solve problems such as restricted directional design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Culture of cells
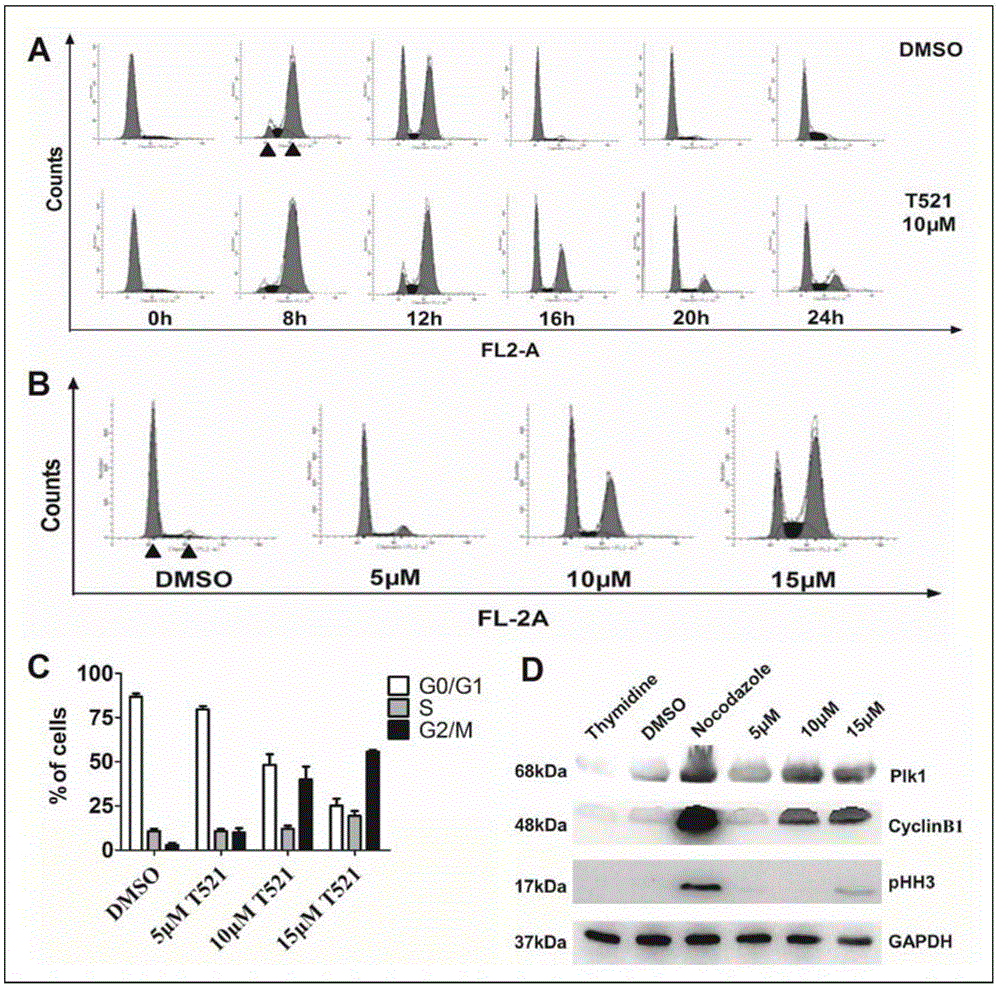
[0054] Human osteosarcoma cell MG63, human osteosarcoma cell SaOS-2, human osteosarcoma cell U-2OS, human bone marrow neuroblastoma cell SH-SY5Y, human breast cancer cell MCF-7, human cervical cancer cell HeLa, human Colon cancer cell HCT-116, human colon cancer cell HT-29, human liver cancer cell HepG2, human liver cancer cell Bel7402, human prostate cancer PC3, human lung cancer A459, human embryonic lung fibroblast MRC-5, human embryonic kidney cell HEK- 293, human hepatocyte L02, all cells are adherent cells, passaged once every 48 hours. After the cells are full, discard the old medium, rinse the cells with PBS and discard, then add an appropriate amount of trypsin, digest at room temperature for about 2-10min, discard the digestion solution, and immediately add the medium containing 10% FBS to Inhibit the activity of trypsin, gently tap the cells in the culture flask repeatedly with an elbow pipette, so that the cells are completely ...
Embodiment 2
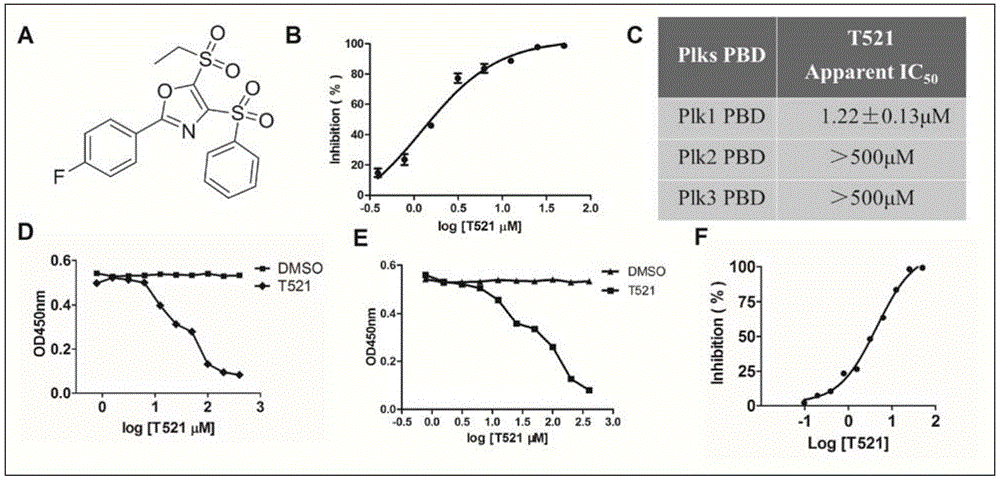
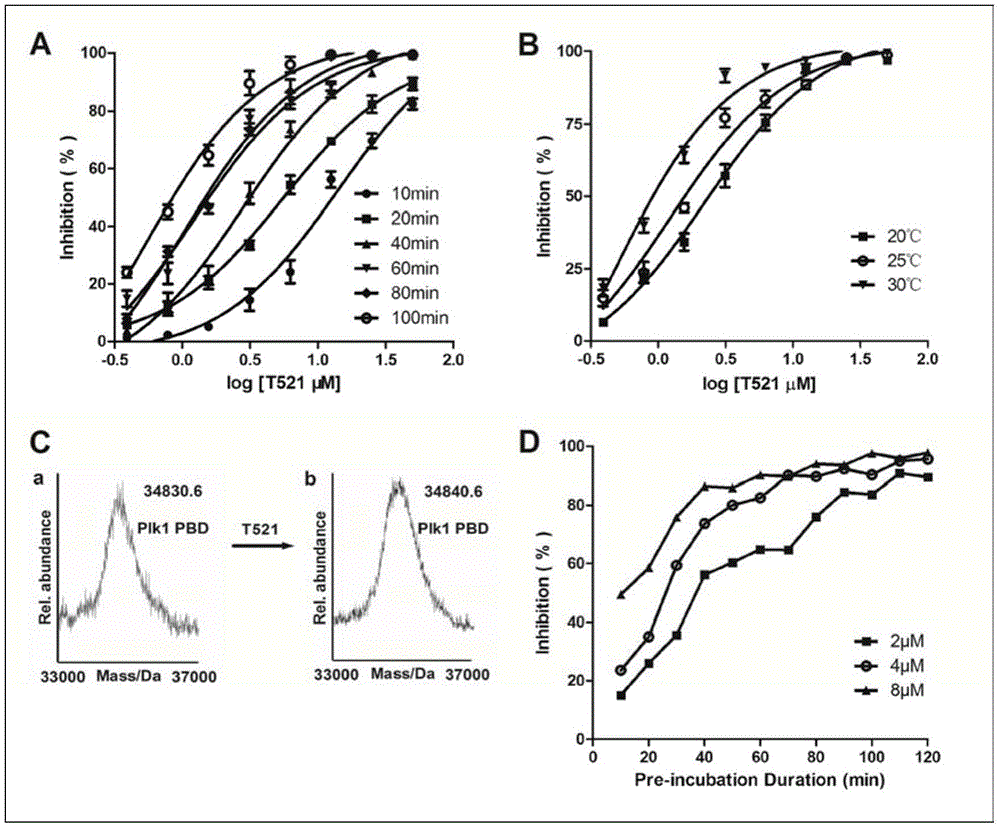
[0055] Example 2 Determination of Compound T521's Inhibitory Activity to PLK1PBD
[0056] The activity assay adopts the fluorescence polarization (FP) high-throughput screening model to measure the activity of the compound.
[0057] Measuring principle:
[0058] It is mainly based on the principle that the polarization of fluorescent molecules is closely related to the rotation speed of fluorescent molecules when they are excited. When a fluorescent molecule is excited by plane polarized light, if the fluorescent molecule remains stationary while being excited, the emitted light will be in the same polarization plane; if the fluorescent molecule is kept rotating while being excited, the emitted light will be in the same polarization plane as the excited light. Different planes of polarization. If fluorescein is excited with vertically polarized light, the intensity of emitted light can be detected in the vertical and horizontal polarization planes (the degree to which the em...
Embodiment 3
[0068] Example 3 Competitive ELISA experiment mediated by compound T521 on Wee1A phosphorylated polypeptide
[0069] The activity was determined by competition ELISA experiment mediated by Wee1A phosphorylated polypeptide.
[0070] Measuring principle:
[0071] Wee1APhosphopeptide (C-EEEGFGSSpSPVKSPAAP-OH) is a synthetic Plk1PBD substrate Wee1 phosphorylation mimic peptide (SpSmotif), which can specifically bind to Plk1PBD protein. The Cysteine sulfhydryl group in Wee1APeptide can covalently react with the maleimide group on the surface of a 96-well plate, thereby immobilizing biomacromolecules or polypeptides containing sulfhydryl groups.
[0072] test methods:
[0073] Coat the plate with 100 μL / well of 4 μM Wee1APeptide and incubate at 37°C for 2 hours; wash 3 times with PBST, 2 minutes each; add 200 μL / well BlockingBuffer to seal, 37°C for 2 hours; Small molecule compound T521 with concentrations of 400 μM, 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM, 3.13 μM, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com