Application of Bufalin-3beta-N-methoxyl-N-beta-D-heteroside in preparing cardiotonic drug
A technology of glucoside and bufalin, which is applied in the field of preparation of cardiotonic drugs, can solve the problems of weak inhibitory effect, and achieve the effect of strong cardiotonic effect, high yield and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
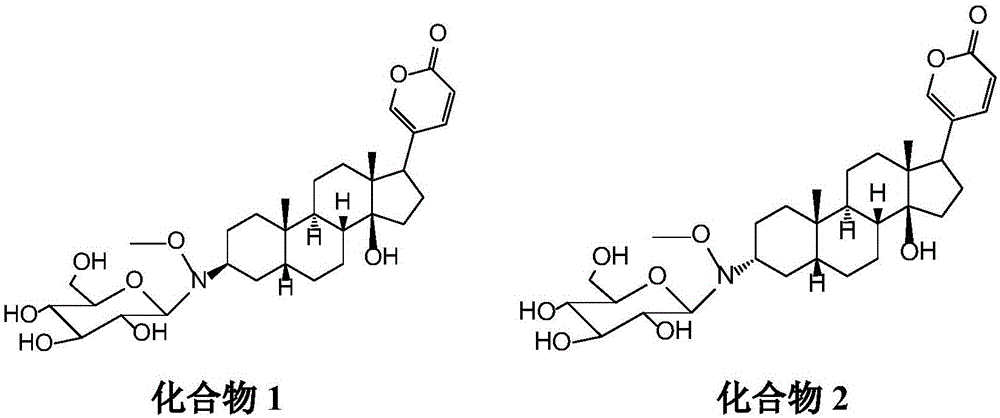
[0024] Experimental example 1: Synthesis of bufalin-3β-N-methoxy-N-β-D-glucoside
[0025] (1) The C3 hydroxyl of the reaction substrate Bufalin is oxidized by the oxidant pyridinium chlorochromate hydrochloride (PCC) to generate the Bufalone of the C3 ketone. This step can be reacted at room temperature and the yield is higher (90 %); (2) the latter reacts with methoxylamine hydrochloride through affinity addition-elimination reaction to generate bufalone derivatives 2a / b, Bufaloneoximes, which have a methoxyl imine structure at the C3 position, and the reaction is comparatively Thoroughly, the yield is higher (yield 90%); (3) oxime mixture Bufaloneoximes is reduced by reducing agent tert-butylamine borane hydrochloride, generates C3 position configuration different isomer compound 3α and 3β, reacts in Carried out under ice-bath cooling, the yield is about 60%, wherein, the intermediate molar ratio is about 3α:3β=2:1, and the two isomers can be separated by silica gel column; ...
Embodiment 2
[0030] Embodiment 2: Bufalin-3β-N-methoxyl-N-β-D-glucoside (compound 1) and bufalin-3α-N-methoxyl-N-beta-D-glucoside ( Compound 2) Na + / K + -ATPase inhibitory activity.
[0031] The test method can be found in literature (Zhang, R.R.; Tian, H.Y.; Tan, Y.F.; Chung, T.Y.; Sun, X.H.; Xia, X.; Ye, W.C., Middleton, M.A.; NatalyaF.; EsmannM.; Tzen, J.T.C.; Jiang, R.W.Structures, chemotaxonomic significance, cytotoxic and Na+, K+-ATPase inhibitory activities of new cardenolides from Asclepiascurassavica.Org.Biomol.Chem.2014, 12(44), 8919-8929.), activity evaluation of compounds 1 and 2, the specific steps are as follows:
[0032] (1) Add the activity test solution to a 96-well plate, 100 μL per well, containing 100mMNaCl, 20mMKCl, 1mMMgCl 2 , 1mMEGTA, 20mMTris-HCl, pH7.4, and 0.2μgofpurifiedNa + / K + - ATPase alpha 1 or alpha 2 subtype.
[0033] (2) After adding different concentrations of compounds 1 and 2, the 96-well plate was incubated at 37° C. for 15 min.
[0034] (3)...
Embodiment 3
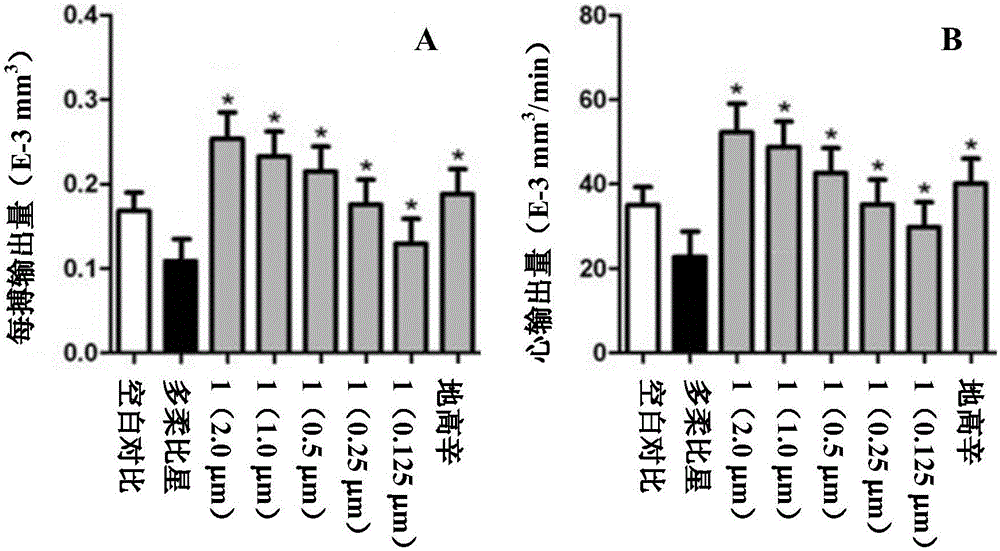
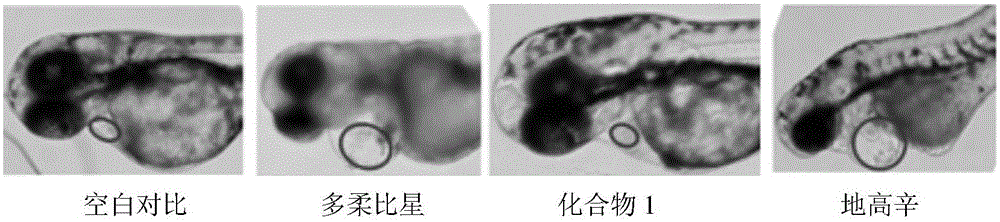
[0037] Example 3: Cardiotonic effect of bufalin-3β-N-methoxy-N-β-D-glucoside (compound 1) on zebrafish
[0038] The present invention uses the zebrafish model with green fluorescence (easy to observe) in the heart to study its cardiotonic effect (Huang, C.C.; Monte, A.; Cook, J.M.; Kabir, M.S.; Peterson, K.P. Zebrafish Heart Failure Models for the Evaluation of Chemical Probes and Drugs. Assay Drug Dev. Technol. , 11(9-10), 561-572.).
[0039] Tg(cmlc2:GFP) transgenic zebrafish (University of Macau) was used, and the fertilized eggs were obtained from mating of male and female fish in the early morning. The fertilized eggs were divided into four groups (medication group, positive control group, negative control group, and vehicle control group), and then the fertilized eggs of each group were placed in the culture medium and hatched at 28°C. After 24 hours, doxorubicin (0.3 μM) was added to the culture medium, and the culture was continued for 24 hours to establish the model)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com