Doped self-assembled nano fiber structure and preparation method thereof
A nanofiber and co-assembly technology, applied in the field of biomedical materials and nanomedicine, can solve the problems of clinical application, poor pharmacokinetics and long-term biological safety of photothermal conversion agents, etc., achieve good tumor specificity, and improve tumor accumulation Efficiency and residence time, effect of high tumor accumulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Can make the present invention be illustrated more clearly below by specific embodiment:
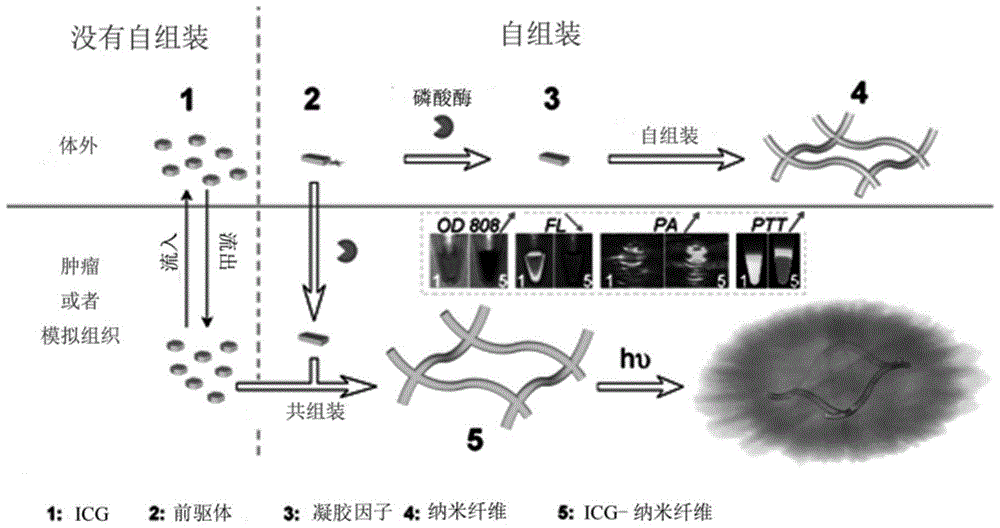
[0029] 1. Preparation of nanofibers:
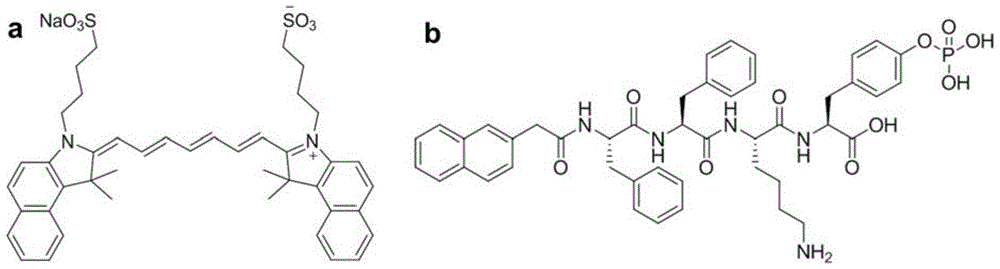
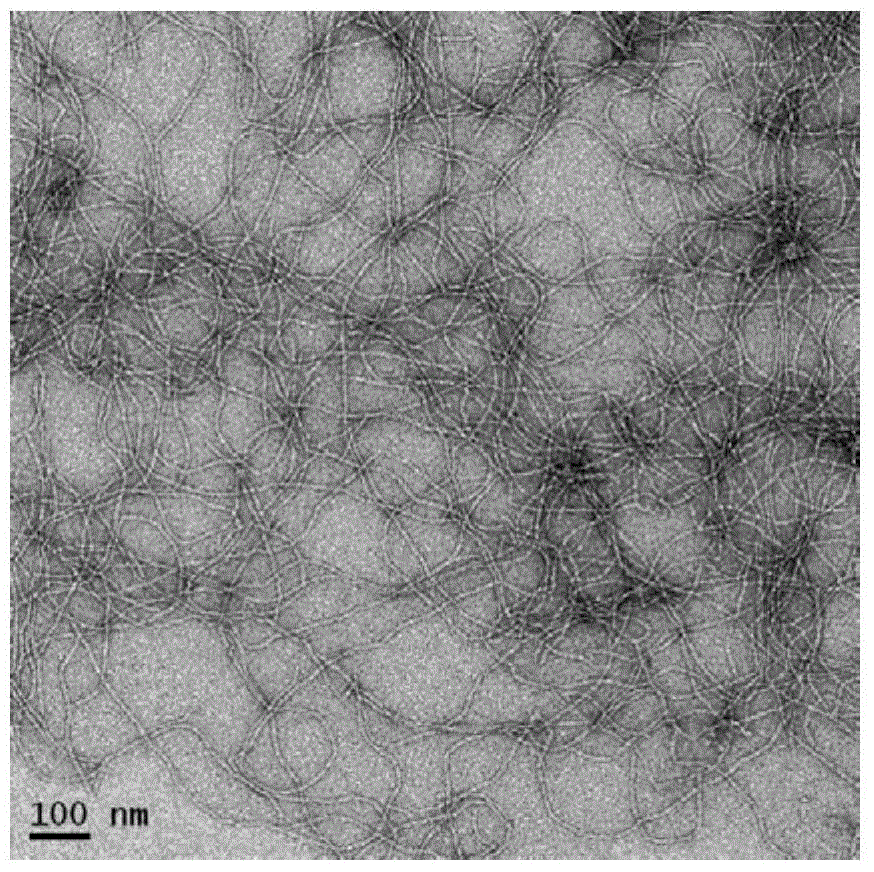
[0030] ICG (10 μg / mL) and NapFFKYp polypeptide (500 μg / mL) were co-dissolved in PBS (PH7-8, 1 mL). ICG-doped nanofibers were formed by adding 1 unit of alkaline phosphatase. Nanofibers without ICG were prepared by the same method as a control.
[0031] 2. Research at the cellular level
[0032] 1) Cultured cells: human-derived cervical cancer cell line HeLa and mouse-derived breast cancer cell line 4T1 were derived from American Type Culture Collection (ATCC), and cultured at 37°C in 5% CO 2 cultured in an incubator.
[0033] 2) Formation of nanofibers in cells: In order to study the formation of nanofibers in cells, 5,000 HeLa cells / well were seeded in a 96-well plate and cultured for 24 hours, and then incubated with different samples: No. 1 sample 10 μg / mL; No. 2 Sample 500 μg / mL; No. 1 sample 10 μg / mL + No. 2 sample 500 μg / mL; No. 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com