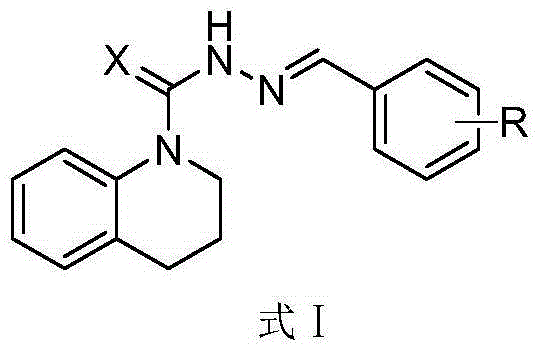
N'-substituted benzal-1,2,3,4-tetrahydroquinoline base-1-(sulfo)hydrazide compound and preparation method and application thereof
A compound, phenoxy technology, applied in the field of pathogenic bacteria control, achieves obvious inhibitory effect, broad-spectrum bactericidal activity, and novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
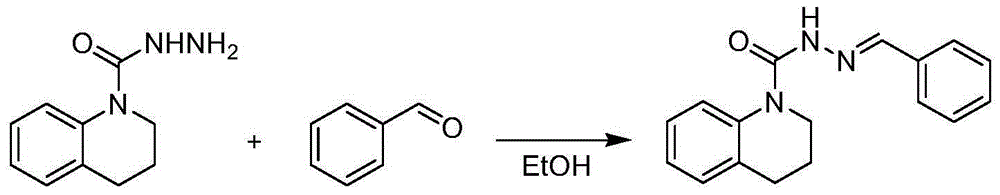
[0025] Add 0.96g (5mmol) of 1,2,3,4-tetrahydroquinolinyl-1-carboxylhydrazide, 0.58g (5.5mmol) of benzaldehyde, and 10mL of absolute ethanol into a 25mL single-necked bottle, and stir at room temperature for 30 minutes. After the reaction was completed, it was suction-filtered and washed with ethanol to obtain 0.94 g of white solid with a yield of 67.2%. The appearance and melting point of this white solid product are shown in Table 1, 1 HNMR spectrum data are shown in Table 2.
Embodiment 2
[0027] Add 0.96g (5mmol) of 1,2,3,4-tetrahydroquinolinyl-1-carboxylhydrazide, 0.63g (6mmol) of benzaldehyde, and 10mL of anhydrous methanol into a 25mL single-necked bottle, and stir at 0°C for 60 minutes. After the reaction was completed, it was filtered with suction and washed with methanol to obtain 0.84 g of white solid with a yield of 59.7%. The appearance and melting point of this white solid product are shown in Table 1, 1 HNMR spectrum data are shown in Table 2.
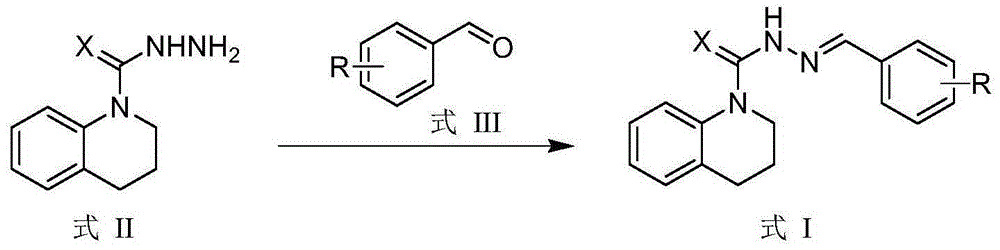
[0028] According to the same method as above for the preparation of compound I-01, only R in formula III is replaced by various substituents, and reacted with the compound shown in formula II to obtain the corresponding products I-02 ~ I-30, the appearance of the compound, Melting point and yield are listed in Table 1, 1 HNMR spectral data are listed in Table 2.
[0029] Preparation of compound N'-(3-methylbenzylidene)-1,2,3,4-tetrahydroquinolinyl-1-thiocarbohydrazide (I-31)
[0030]
Embodiment 3
[0032] In a 25mL single-necked bottle, add 0.41g (2mmol) 1,2,3,4-tetrahydroquinolyl-1-thiocarbohydrazide, 0.26g (2.2mmol) 3-methylbenzaldehyde, 5mL absolute ethanol , stirred at room temperature for 30 minutes. After the reaction was completed, it was suction filtered and washed with ethanol to obtain 0.39 g of a yellow solid with a yield of 62.9%. The appearance and fusing point of this product are shown in Table 1, 1 HNMR spectrum data are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com