Imidazole tri-acylhydrazone compound, application of compound as organic light-emitting material as well as preparation method of compound
A technology of imidazole triacylhydrazone and luminescent materials, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of few varieties of organic luminescent materials and cannot meet application requirements, and achieve reduced interaction, stable structure, The effect of reducing coplanarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of imidazole triacylhydrazone (compound I)
[0033] (1) Preparation of intermediate imidazole tricarboxylate (compound III)
[0034] In a dry 100 ml round bottom flask, add dimethyl 4,4'-(3,4-dioxo-1,5-hexadiene-1,6-diyl)dibenzoate (Compound IV) (0.003mol), methyl 4-formylbenzoate (Compound V) (0.003mol), ammonium acetate (0.03mol), and 30 ml of anhydrous acetic acid were refluxed for 8 hours under rapid stirring. After cooling to room temperature, the reaction solution was poured into ice water under rapid stirring, and the pH was adjusted to 7 with ammonia water. The resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized from a mixed solvent of ethanol-N,N-dimethylformamide and dried in vacuum to obtain a yellow powder with a yield of 71%. The melting point is 178–180°C.
[0035] (2) Preparation of intermediate imidazole triformyl hydrazide (compound II)
[0...
Embodiment 2
[0041] Embodiment 2: the preparation of imidazole triacylhydrazone (compound I):
[0042] (1) Preparation of intermediate imidazole tricarboxylate (compound III)
[0043] In a dry 100 ml round bottom flask, add dimethyl 4,4'-(3,4-dioxo-1,5-hexadiene-1,6-diyl)dibenzoate (Compound IV) (0.003mol), methyl 4-formylbenzoate (Compound V) (0.003mol), ammonium acetate (0.03mol), and 30 ml of anhydrous acetic acid were refluxed for 8 hours under rapid stirring. After cooling to room temperature, the reaction solution was poured into ice water under rapid stirring, and the pH was adjusted to 7 with 10% aqueous sodium hydroxide solution. The resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallize from a mixed solvent of ethanol-N,N-dimethylformamide, and dry in vacuo to obtain a yellow powder. The melting point is 178–180°C.
[0044] (2) Preparation of intermediate imidazole triformyl hydrazide (compound II)
...
Embodiment 3
[0048] Embodiment 3: Fluorescence performance test of imidazole triacylhydrazone (compound I)
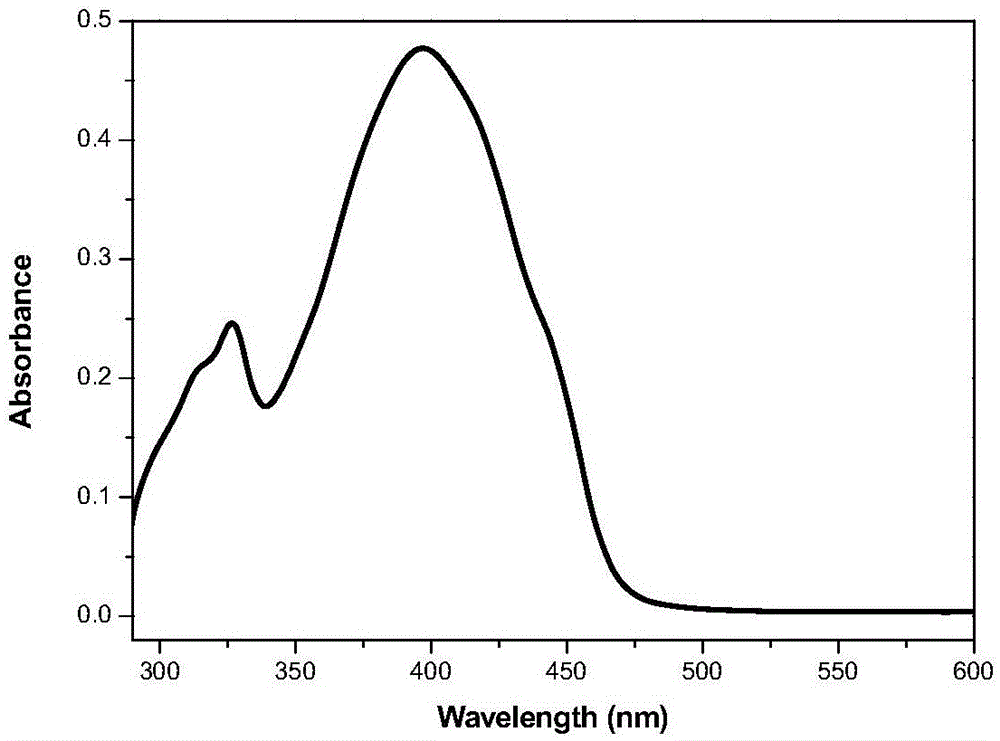
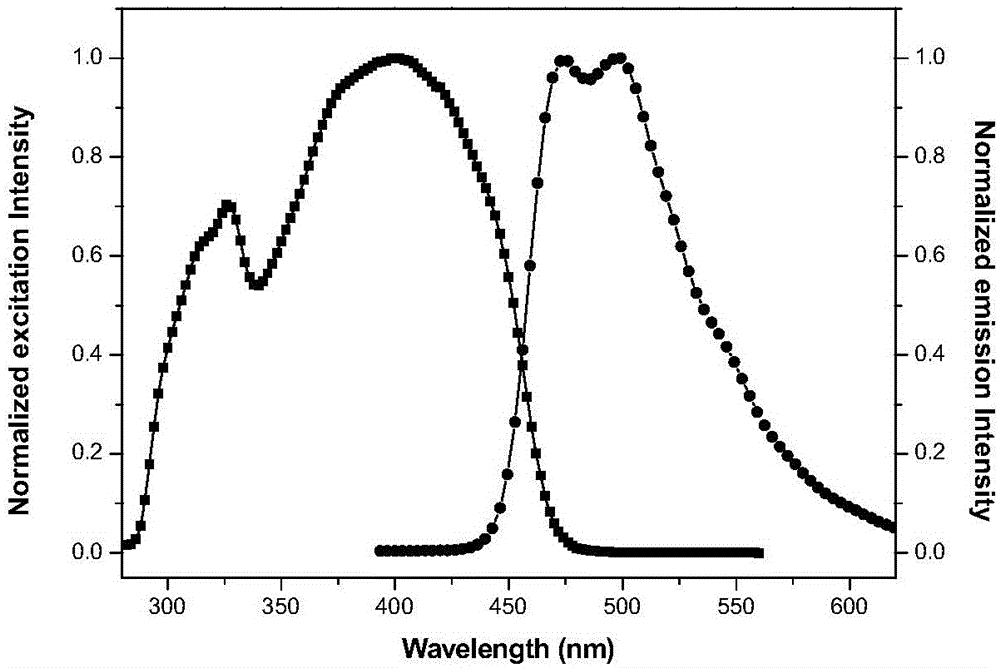
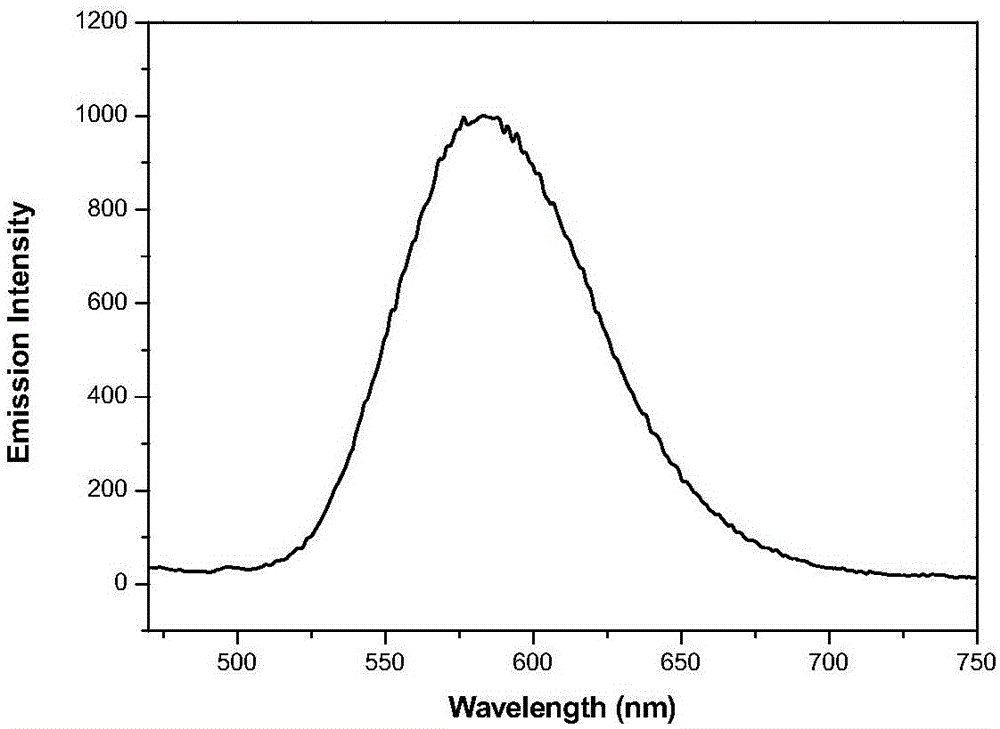
[0049] It is 2 * 10 that the compound of embodiment 1 is formulated into concentration -5 M tetrahydrofuran solution. Measure its ultraviolet absorption and fluorescence properties on a HORIBA JobinYvonAqualog absorption and three-dimensional fluorescence scanning spectrometer with a 1 cm fluorescence cell, the results are as follows figure 1 and 2 shown. The solid-state fluorescence spectrum is measured with a PerkinElmer LS-55 fluorescence spectrophotometer, the results are shown in image 3 .
[0050] Simultaneously observe the fluorescent color of the solid powder of Compound I obtained in Example 1 and its tetrahydrofuran solution under the irradiation of a 365 nm ultraviolet lamp. The results show that under the irradiation of 365nm ultraviolet lamp, compound I emits strong blue-green fluorescence in solution, and emits strong yellow fluorescence in solid state.
[0051]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com