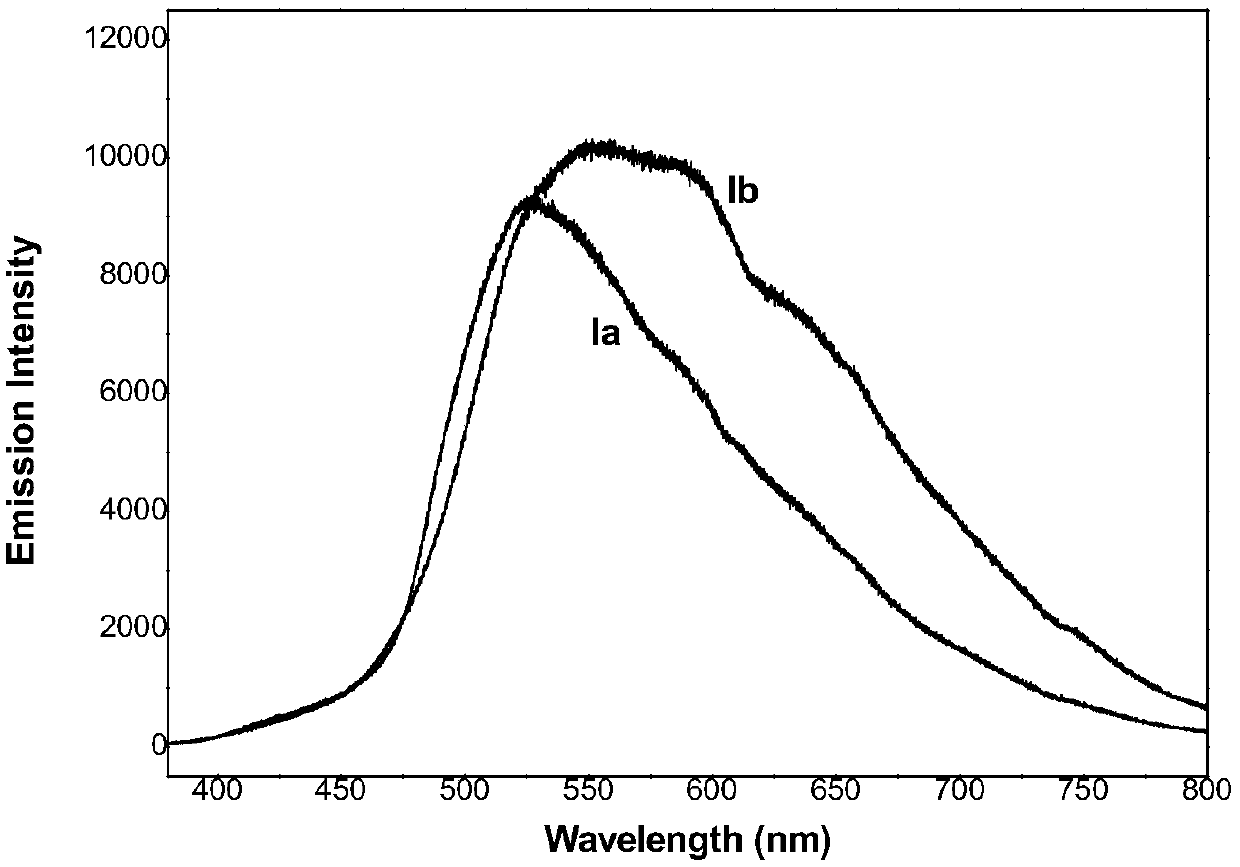
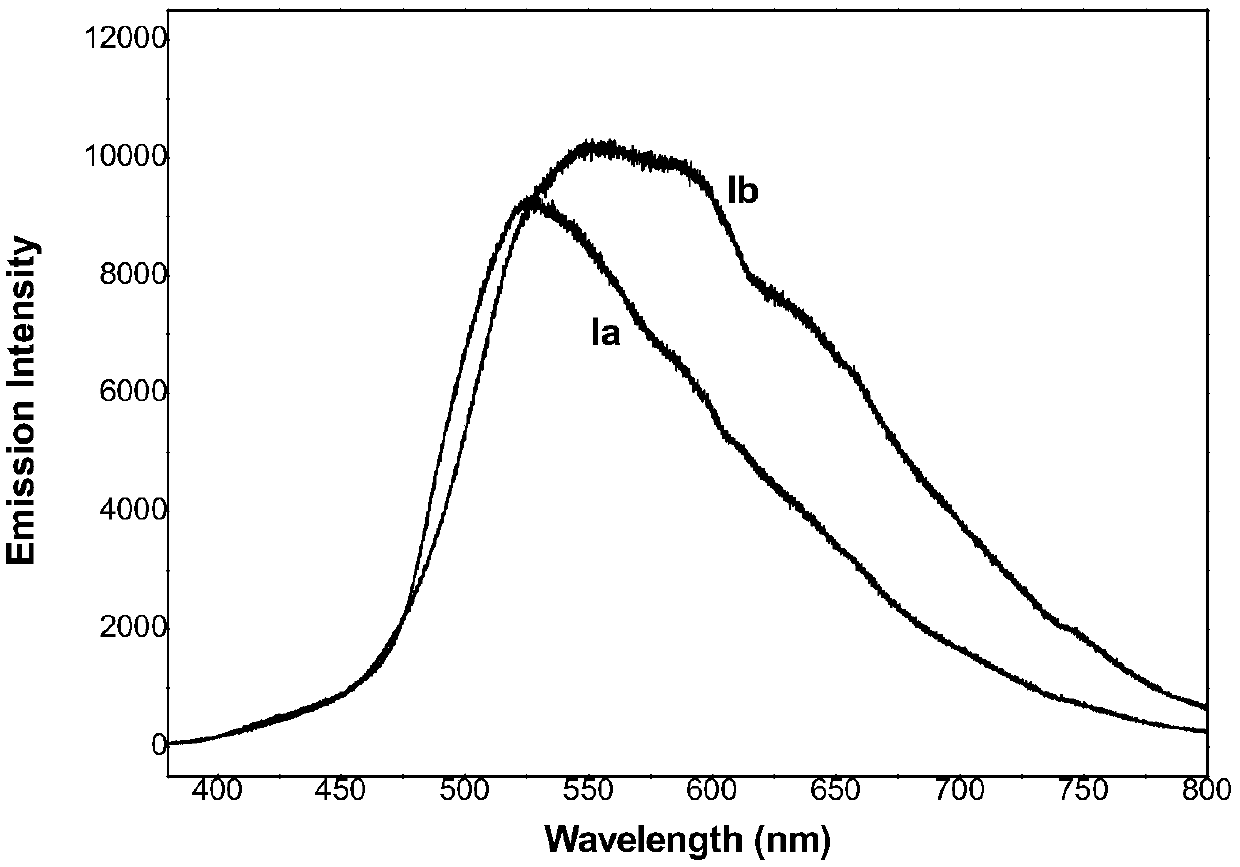
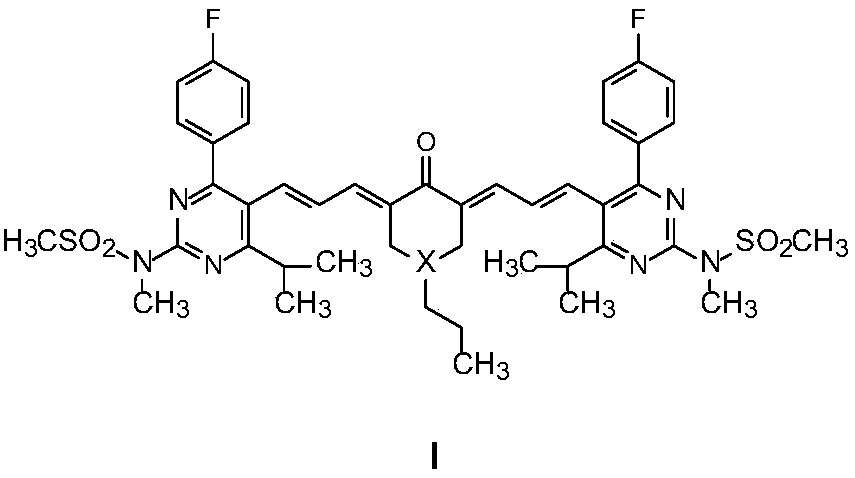
A kind of fluorophenylpyrimidine solid-state green light-emitting organic luminescent material and preparation method thereof
A technology of substituted phenylpyrimidines and luminescent materials, which is applied in the field of fluorophenylpyrimidine-based solid-state green light-emitting organic luminescent materials and their preparation, to achieve the effects of increasing solubility, preventing fluorescence quenching, and improving processing performance and practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 4-propyl-2,6-bis(3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidine Preparation of -5-yl) allyl)-cyclohexanone (compound Ia)
[0029]
[0030] In a 250 ml round bottom flask, mix 4-propylcyclohexanone (1 mmol) and 3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methyl Sulfonylamino) pyrimidin-5-yl) acrolein (2mmol) is dissolved in 100 milliliters of ethanol, and in this solution, dripping 20 milliliters of mass fractions is 15~20% potassium hydroxide solution under rapid stirring, and at room temperature The reaction was stirred for 10-15 hours. Afterwards, the reaction solution was poured into 150 ml of water and left to stand, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized with ethanol-acetone mixed solvent and dried in vacuum to obtain yellow-green needle crystals with a yield of 78%.
[0031] 1 H NMR (300MHz, CDCl 3 / TMS) δ: 0.9...
Embodiment 2
[0032] Example 2: 1-propyl-3,5-bis(3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidine Preparation of -5-yl)allylylene)-4-piperidone (compound Ib):
[0033]
[0034] In a 250 ml round bottom flask, mix 4-propylcyclohexanone (1 mmol) and 3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methyl Sulfonylamino) pyrimidin-5-yl) acrolein (2mmol) is dissolved in 100 milliliters of methanol, and 20 milliliters of sodium hydroxide solution that the mass fraction is 15~20% is added dropwise to this solution under rapid stirring, and at room temperature The reaction was stirred for 10-15 hours. Afterwards, the reaction solution was poured into 150 ml of water and left to stand, and the resulting solid was filtered under reduced pressure, washed with water several times, and dried at room temperature. Recrystallized with ethanol-acetone mixed solvent and dried in vacuum to obtain yellow flaky crystals with a yield of 72%.
[0035] 1 H NMR (300MHz, CDCl 3 / ...
Embodiment 3
[0036]Example 3: 4-propyl-2,6-bis(3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidine Preparation of -5-yl) allyl)-cyclohexanone (compound Ia)
[0037] Obtained by the method of Example 1, the difference is that 4-propyl cyclohexanone and 3-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methyl The molar ratio of sulfonylamino)pyrimidin-5-yl)acrolein is 1:2.3, and the recrystallization solvent used is ethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com