Polypeptides having bradykinin receptor binding activity and uses thereof
A technology of receptor binding and bradykinin, which is applied in the fields of pharmacy and biology, can solve the problems of poor stability of L-type polypeptides, achieve the effects of increasing accumulation, prolonging median survival, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1RI-B
[0029] Embodiment 1RI-BK, the preparation and characterization of RI-KD and RI-L-BK
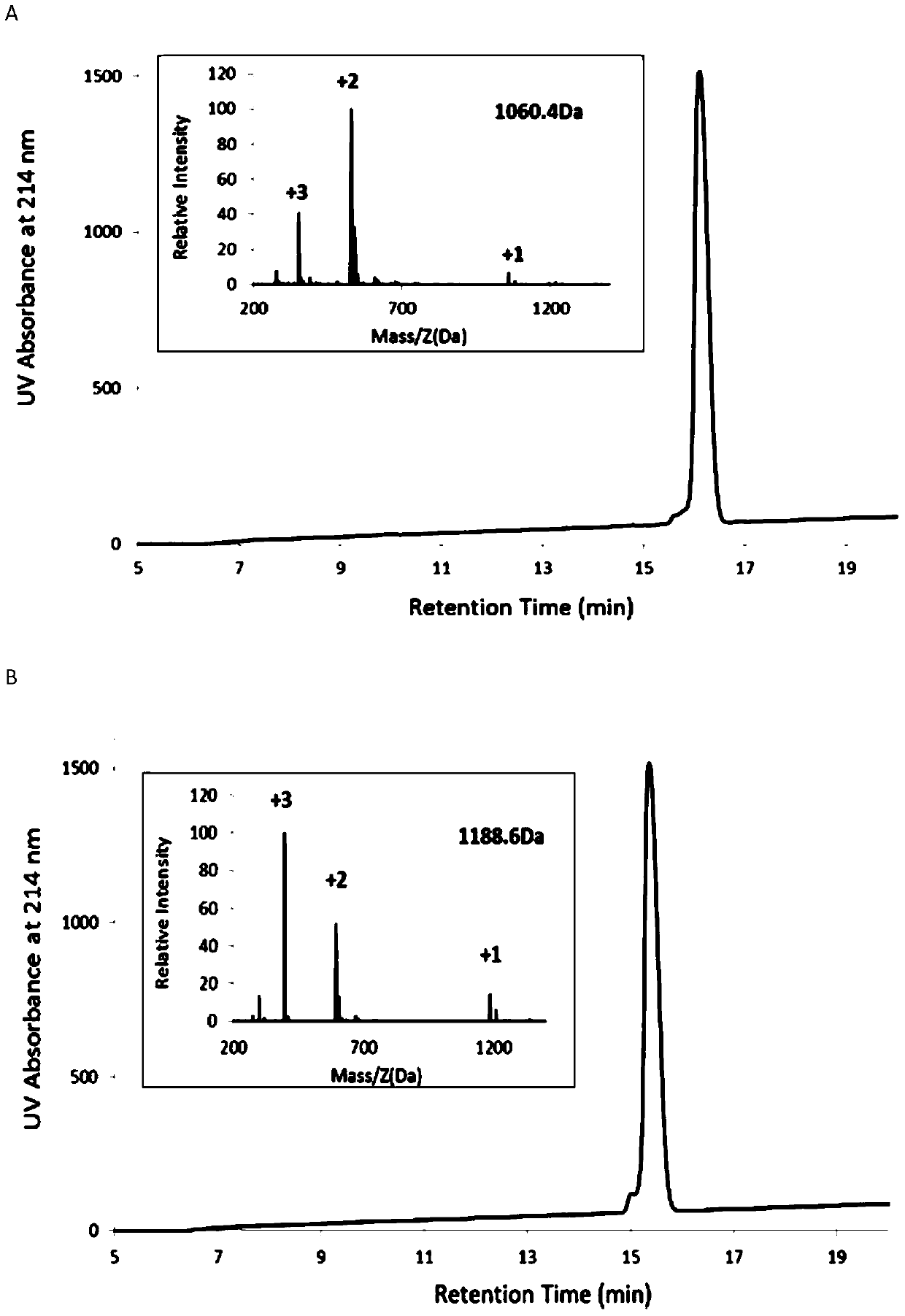
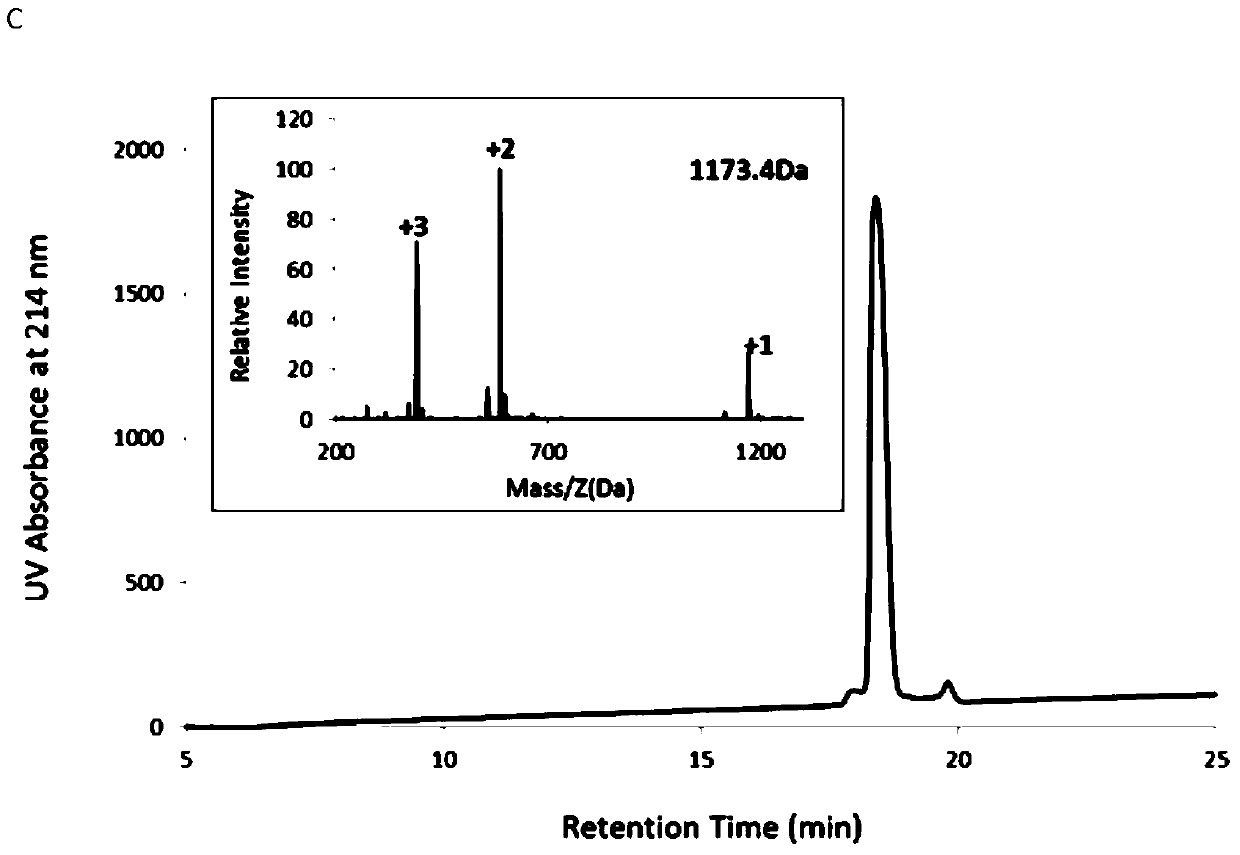
[0030] Using the solid-phase synthesis method, deprotect the PAM-Boc resin with trifluoroacetic acid (TFA) for 1 minute, twice, and react with Boc-protected amino acids in turn. Wash the resin with / MeOH (1 / 1), dry it in vacuum, put the resin into a polypeptide cutting tube, add an appropriate amount of P-cresol, then pass through HF, stir and react in an ice bath for 1 hour, and remove the HF in the tube under reduced pressure after the reaction is completed. The precipitate was washed with glacial ether for 3 times, the residual precipitate was dissolved in 20% acetonitrile and then rotary evaporated, separated and purified with acetonitrile / water (containing 0.1% TFA) system, HPLC and ESI-MS were used to characterize RI-BK, RI-KD and RI-L- The purity and molecular weight of BK; Its HPLC collection of illustrative plates and mass spectrogram are as figure 1 As shown, the T of RI-BK RI-BK ...
Embodiment 2
[0032] Uptake of RI-BK-FITC by C6, HEK, U87, HUVEC and bEnd.3 cells
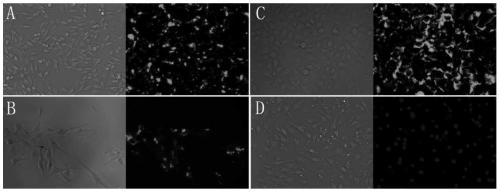
[0033] C6, U87, HUVEC, and bEnd.3 cells were inoculated in 12-well plates, and 24 hours after the cells adhered, the DMEM containing serum was discarded, and DMEM without serum and RI-BK-FITC fluorescein mother solution were added to each well. , the final concentration of fluorescein was controlled to be 5×10 -6 mol / L. Continue culturing for 2 hours, take it out, wash twice with PBS, fix with formaldehyde for 15 minutes, wash twice with PBS, stain with DAPI for 10 minutes, wash twice with PBS, add buffered glycerol at the end, and take photos with a fluorescent microscope. The test results show that C6, U87, HUVEC Both have significant uptake of RI-BK-FITC, and bEnd.3 has basically no uptake of RI-BK-FITC (such as figure 2 shown).
Embodiment 3
[0035] RI-BK, RI-KD and RI-L-BK inhibited the uptake of RI-BK-FITC in C6 cells by fluorescence photos and flow cytometry results
[0036] C6 cells were seeded in 12-well plates. After the cells adhered to the wall, the DMEM containing serum was discarded, and DMEM without serum and polypeptides RI-BK, RI-KD and RI-L-BK were added to each well to control the concentration of the polypeptides. The final concentration was 5×10 -3 mol / L and incubated for half an hour, then add RI-BK-FITC fluorescein mother solution to control the final concentration of fluorescein to be 5×10 -6 mol / L; take it out after continuing to culture for 2 hours, wash twice with PBS, fix with formaldehyde for 15 minutes, wash twice with PBS, stain with DAPI for 10 minutes, wash twice with PBS, and finally add buffered glycerol; take pictures under a fluorescent microscope; the test results are as follows image 3 At the same time, trypsinized C6 cells, centrifuged at 1000 rpm for 5 minutes, washed once wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com