ADP-glucose pyrophosphorylase mutant and screening method and application thereof
A phosphorylase mutant, glucose coke technology, applied in biochemical equipment and methods, applications, botanical equipment and methods, etc., can solve the problem of insufficient attention to research on high starch varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
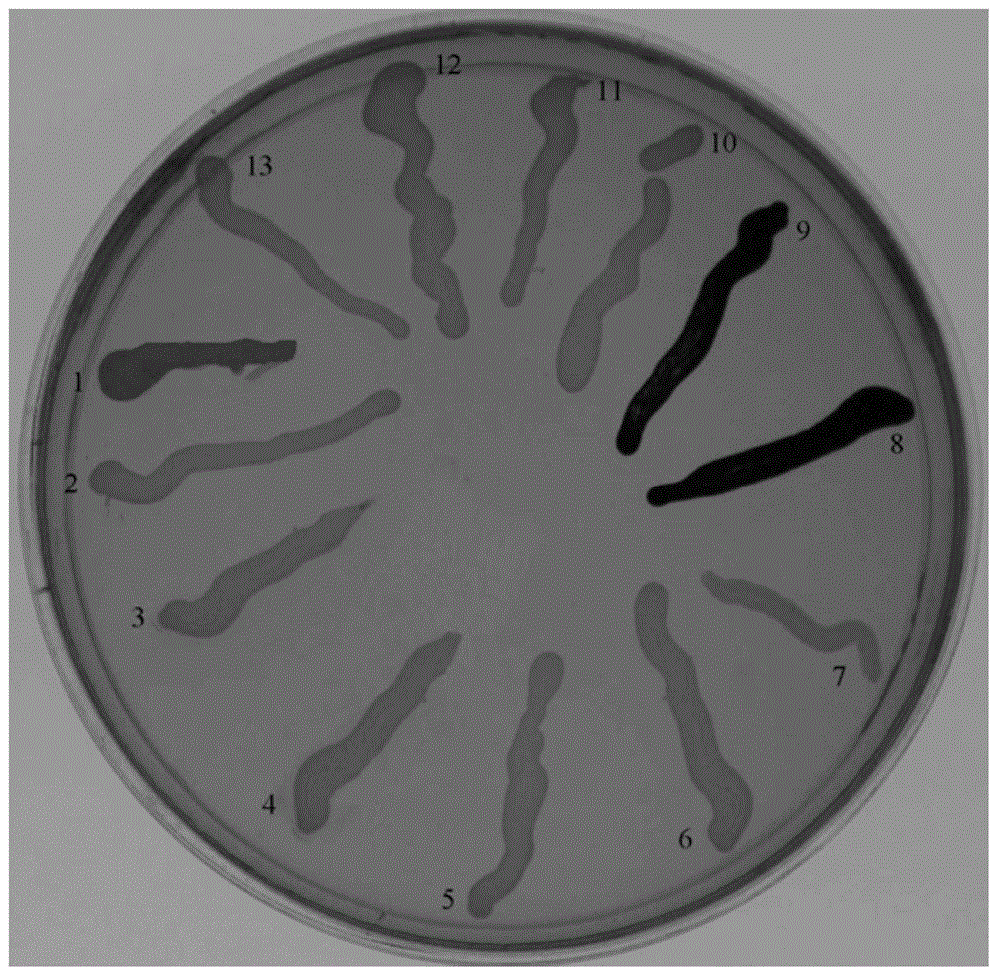
Examples
Embodiment 1
[0079] Embodiment 1: prepare the prokaryotic expression vector of the large and small subunit cDNA recombinant gene of AGPase
Embodiment 1a
[0080] Example 1a: Obtaining of large and small subunit cDNA of corn endosperm and sweet potato AGPase
[0081] (1) Primer design
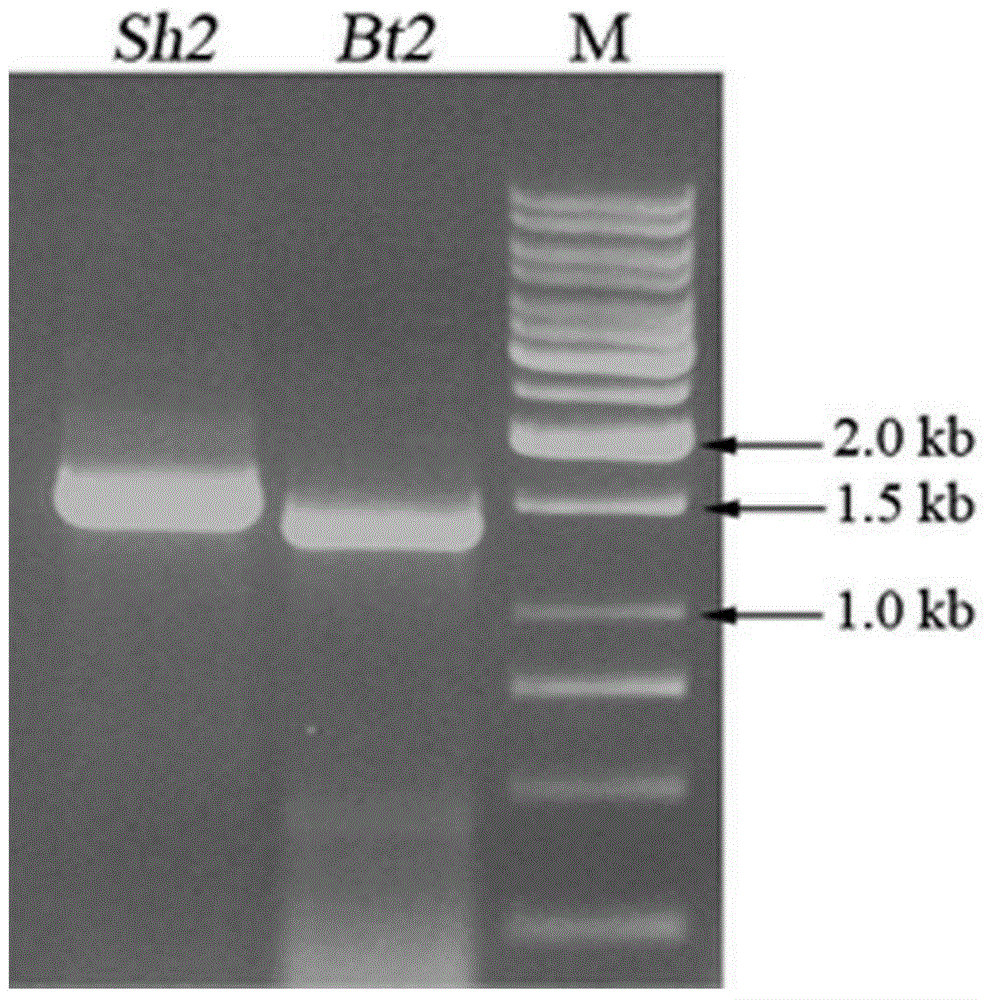
[0082] According to the cDNA sequences of the large subunit Shrunken-2 and the small subunit gene Brittle-2 of maize endosperm AGPase (GenBank numbers are: M81603, AF334959) and the cDNA sequences of the large and small subunits of sweet potato AGPase (GenBank numbers are: AB271013.1, AB271014 .1, AB271015.1, AB271016.1, AB271011.2, AB271012.1) designed RT-PCR primers; at the same time, in order to facilitate the construction of the vector, a special design was made when designing the primers: 5 The recognition site of restriction endonuclease NdeI is added at the 'end, and the recognition site of restriction endonuclease KpnI is added at the 3' end; SacI recognition site of endonuclease, the primer sequence is as follows:
[0083] The primer pair for amplification of the large subunit Shrunken-2 cDNA of maize endosperm AGPase is as follows:
...
Embodiment 1b
[0137] Embodiment 1b: Construction of AGPase large and small subunit cDNA prokaryotic expression vector
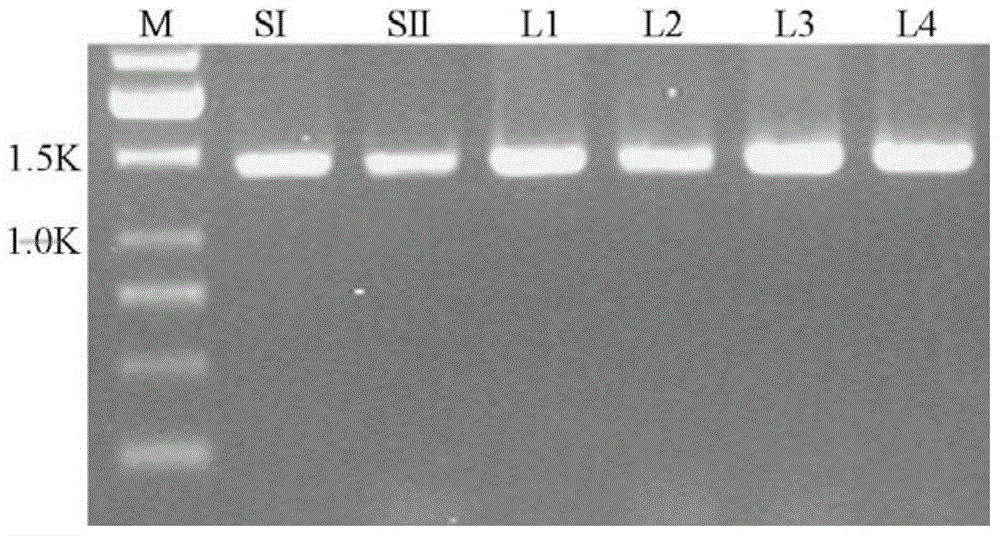
[0138] The AGPase large subunit Shrunken-2 cDNA was double-digested with restriction endonucleases NdeI and KpnI, and then connected to the prokaryotic expression vector that had been double-digested with the same enzymes to obtain a recombinant vector containing the AGPase large subunit Shrunken-2 cDNA; Sweet potato small subunit SIcDNA was double-digested with restriction endonucleases NcoI and SacI, and then ligated with the recombinant vector of the above-mentioned AGPase large subunit Shrunken-2 at a molar ratio of 1:1 to obtain the recombinant cDNA containing the large and small AGPase subunit Gene prokaryotic expression vector, referred to as SSI;
[0139] The AGPase large subunit Shrunken-2 cDNA was double-digested with restriction endonucleases NdeI and KpnI, and then connected to the prokaryotic expression vector that had been double-digested with the same enzyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com