Indometacin amorphous particle, particle drug preparation, preparation method and application
An indomethacin and amorphous technology, applied in the field of indomethacin amorphous particles, can solve the problems of poor dissolution, low oral bioavailability, and low dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
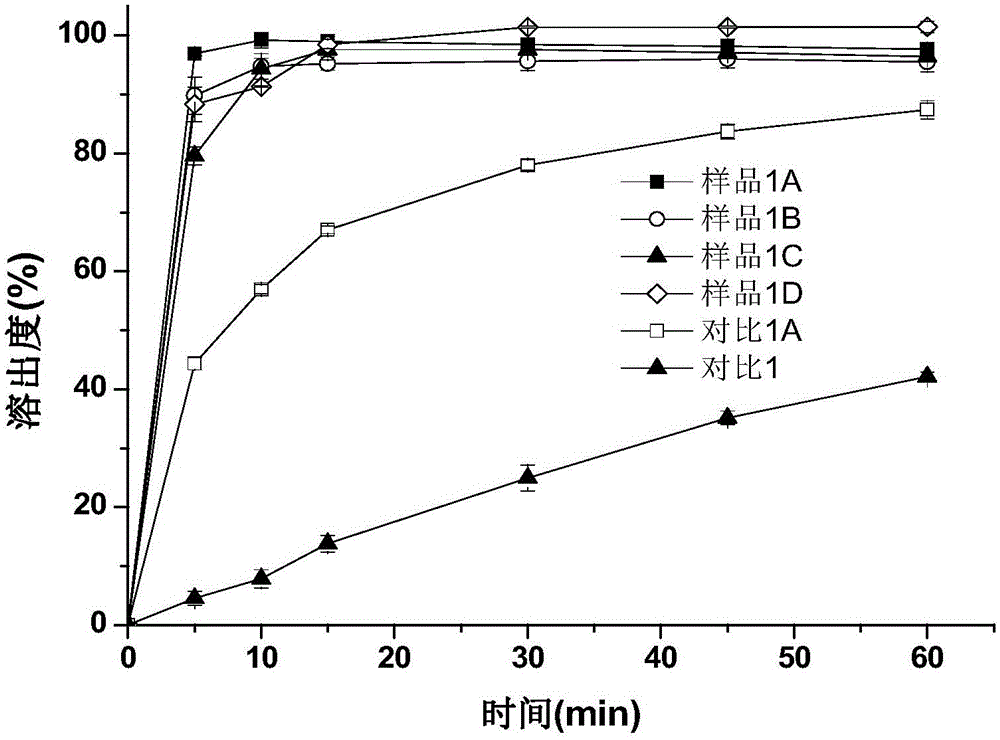
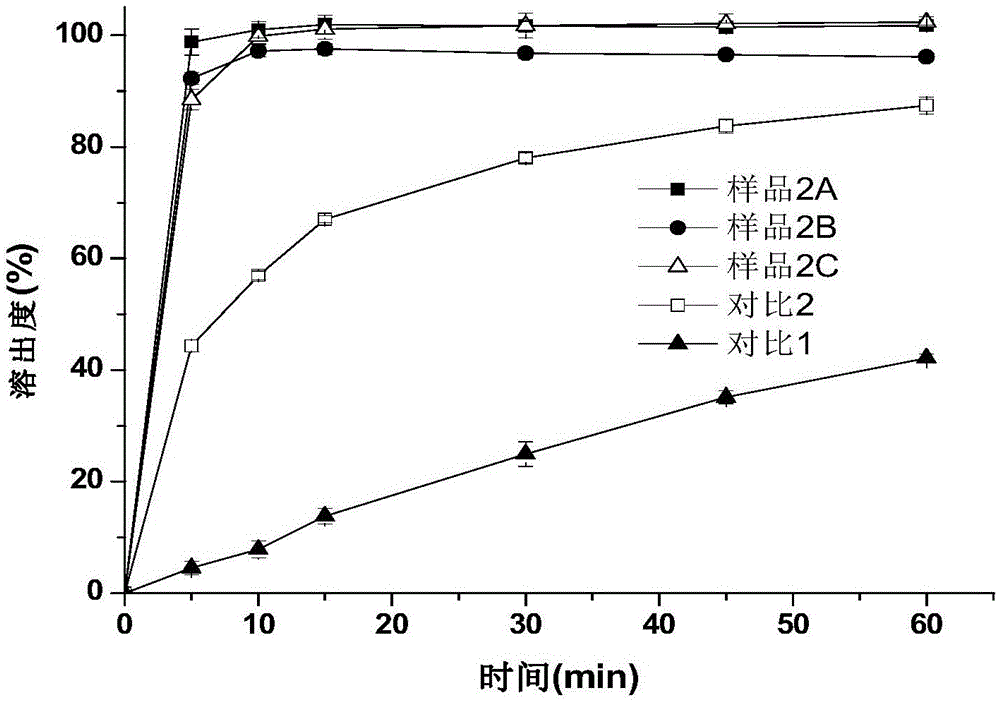
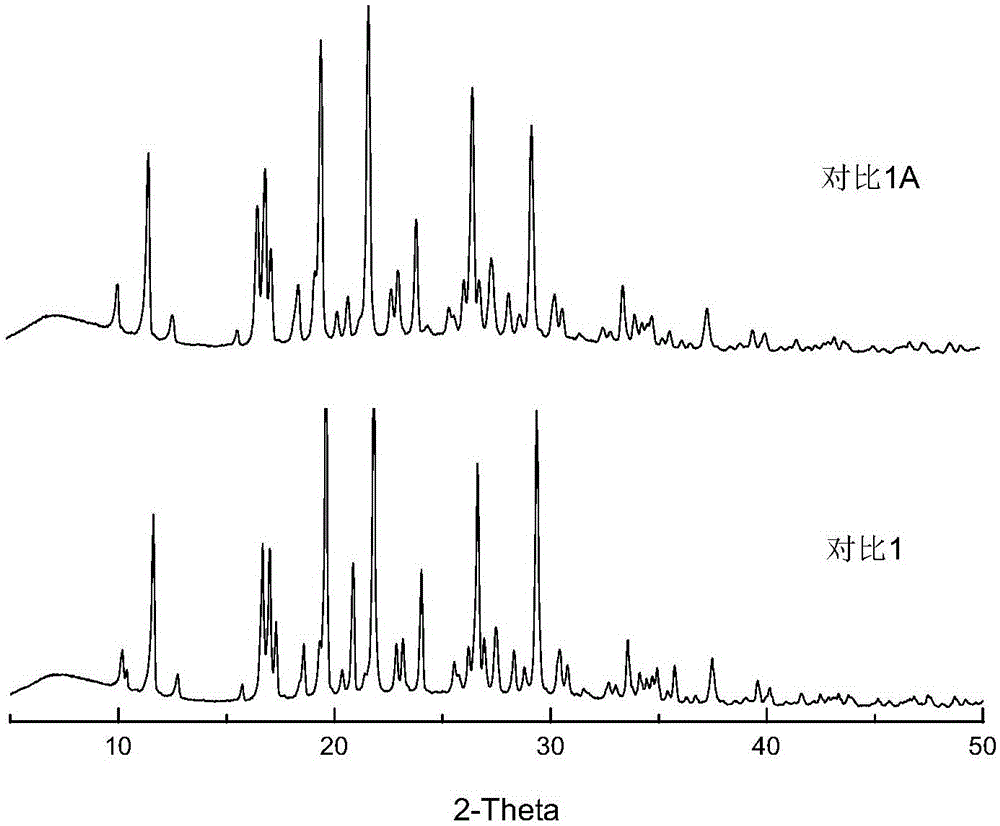
[0064] Get 1.5g of indomethacin bulk drug at room temperature, add 250mL of pH6.0 phosphate buffer (take 1.2g of sodium dihydrogen phosphate and add 800ml of water to dissolve, take a mass concentration of 0.4% sodium hydroxide solution to adjust the pH to 6.0, Add water to 1000ml to obtain), 600rpm magnetic stirring to disperse, then add 0.075g povidone K30, and slowly add a mass concentration of 4% NaOH solution to dissolve it with a peristaltic pump (the mass concentration refers to the mass of sodium hydroxide Accounting for the percentage of the total mass of sodium hydroxide solution), the pH is controlled to be 8.9, and a small amount of insoluble matter is filtered out. Under the condition of 25°C, a peristaltic pump was used to drop a HCl solution with a mass concentration of 3.65% at a speed of 1 rpm, the indomethacin amorphous particles were gradually precipitated, and the drop was stopped until the pH of the mixed solution was 3.0. Put the mixed solution of indomet...
Embodiment 2
[0070] Under room temperature, get 1.5g indomethacin crude drug, add 250mL, the phosphate buffer solution of pH6.5 (get 1.2g sodium dihydrogen phosphate and add 800ml water to dissolve, get mass concentration and be 0.4% sodium hydroxide solution to adjust pH to 6.5, add water to 1000ml to obtain), 600rpm magnetic stirring to disperse, then add 0.075g hypromellose (viscosity is 5mPa.s), and slowly add mass concentration of 4% NaOH solution to dissolve it with a peristaltic pump, and control pH8-9, filter out a small amount of insoluble matter. At 30°C, a peristaltic pump was used to drop a HCl solution with a mass concentration of 3.65% at a speed of 1 rpm, the sample was gradually precipitated, and the drop was stopped until the pH of the mixed solution was 3.0. After standing for a period of time, the mixed solution of indomethacin amorphous particles was suction-filtered, washed 3 times with water, and the sample was collected, dried in an oven at 40°C for 10 hours, ground,...
Embodiment 3
[0075] Get 1.5g indomethacin crude drug at room temperature, add 150mL pH7.0 citrate buffer solution containing 10% ethanol (get 2.9g sodium citrate dihydrate and add 800ml water to dissolve, take mass concentration and be 4% Sodium hydroxide solution to adjust the pH to 7.0, add water to 1000ml to obtain), 300rpm magnetic stirring to disperse, then add 0.3g povidone K-30, slowly add the mass concentration with a peristaltic pump to a mass concentration of 4% NaOH solution to control the pH To 7.5-8.5 to dissolve it. At 25°C, a peristaltic pump was used to drop HCl solution with a mass concentration of 3.65% at a speed of 1 rpm, particles containing amorphous indomethacin were gradually precipitated, and the drop was stopped until the pH of the mixed solution was 3.5. The mixed solution containing the particles of amorphous indomethacin was left to stand for a period of time, then suction filtered, washed 3 times with water, and the sample was collected, dried in an oven at 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com