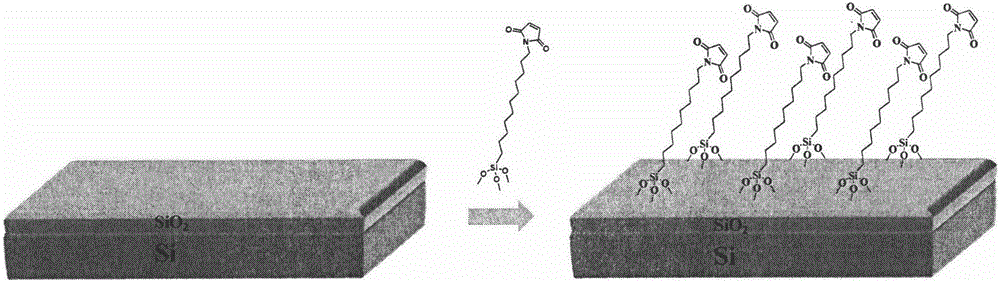
Synthesis of maleimidotriethoxy silane-series compounds, and preparation method of self-assembled film
A technology of maleimidotriethoxysilane and its synthesis method, which is applied in the fields of chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., and can solve the problem of catalytic yield and repeatability Poor and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of self-assembly material 11-maleimidoundecyltriethoxysilane.
[0027]
[0028] Synthesis of 10-undecylenamide:
[0029]
[0030] Take urea (15g, 0.25mol) and undecylenic acid (23.01g, 0.125mol) in a two-necked flask, raise the temperature to 170-180°C, and stop heating after 4 hours of reaction. When the temperature drops to 110-120°C, add 5% Na 2 CO 3 The solution (100-150ml) was used to stop the reaction, and the mixture was placed in an ice-water bath, filtered after solid precipitation, recrystallized twice with 95% ethanol, and vacuum-dried to obtain a colorless solid with a yield of 60%. 1 HNMR (400MHz; CDCl 3 ): δ5.80 (1H, m), 5.47 (2H, s), 2.22 (2H, t), 2.04 (2H, m), 1.63 (2H, m), 1.38-1.29 (10H, m).
[0031] Synthesis of 11-amino-1-undecene:
[0032]
[0033] Take LiAlH 4 (2.3g, 60.6mmol), 50ml of dry THF in a 250ml two-necked flask, protected by nitrogen, heated to reflux for 30min and then stopped heating. Compound 10-undecylena...
Embodiment 2
[0041] (1) Preparation of self-assembled membrane
[0042] Step 1: Wash the silicon wafer with a size of 1cm×1cm with a neutral detergent and wash it with a large amount of ultrapure water;
[0043] Step 2: Sonicate silicon wafers with acetone, ethanol, and ultrapure water for 10 minutes in sequence and use N 2 blow dry;
[0044] Step 3: Incorporate the cleaned Si / SiO 2 The substrate is immersed in the piranha solution (H 2 SO 4(conc.) / H 2 o 2(30%) =5:1) for 24h to form hydroxyl groups on its surface;
[0045] Step 4: Dip the activated silicon wafer in 10ml of toluene (dry) solution of 11-maleimidoundecyltriethoxysilane (10 -3 M), N 2 protected, heated to 75°C, and self-assembled for 48h, the Si / SiO 2 A self-assembled film of 11-maleimido undecyltriethoxysilane is self-assembled on a substrate.
[0046] (2) Characterization of materials
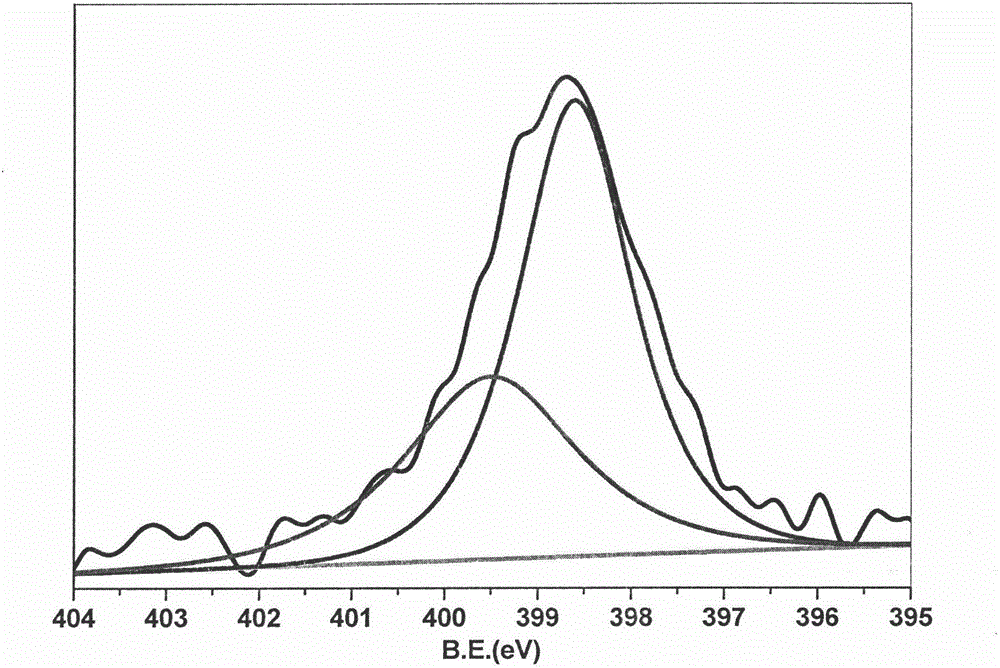
[0047] (a) X-ray photoelectron spectroscopy (XPS)
[0048] figure 2 It is the XPS schematic diagram of N1s. The peak with bin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com