Preparation method and application of supported noble metal catalyst
A noble metal catalyst, supported technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, chemical instrument and method, physical/chemical process catalyst, etc. Complex precious metal process and other issues, to achieve the effect of reducing the sintering probability of precious metals, reducing the sintering probability, and outstanding anti-aging ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preferred embodiment of the present invention, the preparation method of supported noble metal catalyst comprises the following steps:
[0036] (1) Preparation of noble metal precursor solution.
[0037]Weigh the corresponding mass of soluble precious metal raw material, and add 0.5-5 times the volume of solvent of incipient wetness water pore volume. Adjust the pH at -0.5 to 12. Add a protective agent with an amount of 0-10 times the precious metal (based on the molar mass of the monomer). Stir well.
[0038] (2) The precious metal and the carrier are evenly mixed.
[0039] Add the precursor solution to the carrier in spray form or add the carrier to the precursor solution. Stir at 0-90°C for 0.5-8h.
[0040] (3) Add protective agent.
[0041] Weigh the protective agent (calculated as monomer) that is 0 to 10 times the stoichiometric amount of the precious metal and dissolve it in the suspension that has been uniformly mixed in step (2). Stir at 0-90°C for 0.5...
Embodiment 1
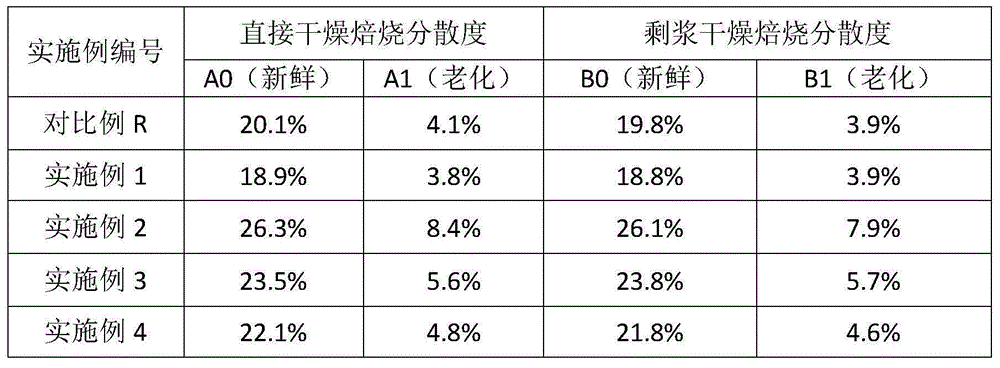
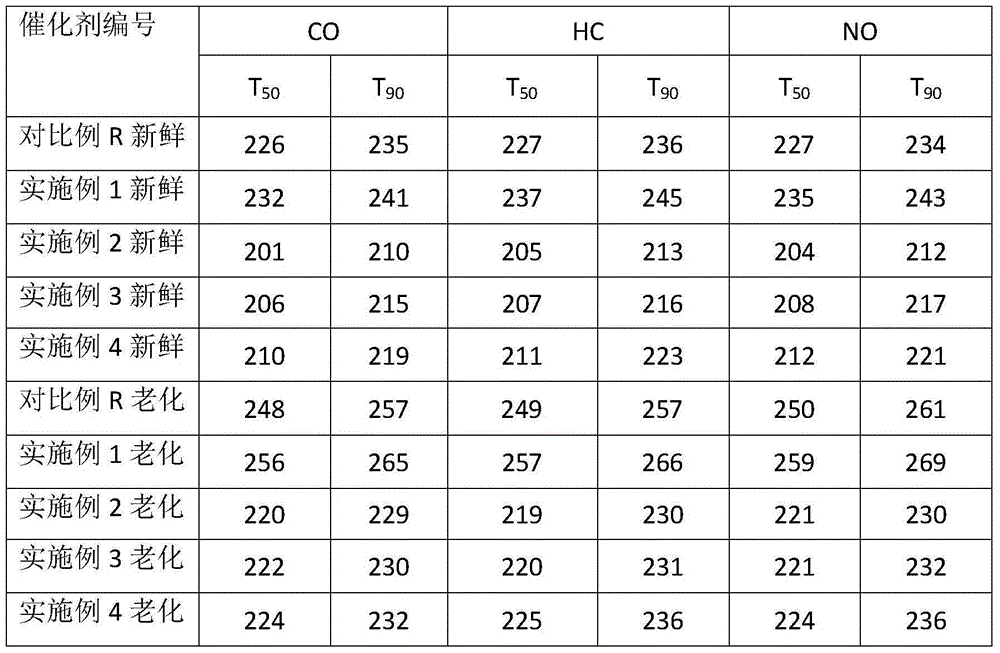
[0052] Use dilute ammonia water to adjust the pH of the palladium nitrate solution to 1.0, add deionized water to make the volume of the precursor solution 0.6 times the volume of the water pores of the carrier, add it to the Ce / Zr carrier in the form of a spray, stir rapidly at room temperature until uniform, and statically set for 2h. Weigh the PVP with a metering ratio of 1:1 and dilute it to 0.2 times the volume of the water pores, then add it in the form of a spray and stir it quickly until it is uniform, and let it stand for 0.5h. Add 0.2 times the volume of methanol in the water pores, add it in the form of a spray and stir it quickly until it is uniform, and let it stand for 2h. After a part was dried at 110°C for 2 hours, it was calcined at 550°C for 2 hours, and a sample was taken to test the dispersion degree of the precious metal, which was recorded as the dispersion degree A0. Part of it was directly added with deionized water and binder, and ball milled for 15 m...
Embodiment 2
[0054] Prepare 5g / L palladium nitrate solution, add dilute ammonia water dropwise to control the pH at 1.0. Add Ce / Zr carrier, stir in 80°C water bath for 4h. Add PVP (measurement ratio 1:1), and stir in a water bath at 80°C for 1h. Then add methanol with a metering ratio of 1:4, and stir in a water bath at 80°C for 4h. After a part was dried at 110°C for 2 hours, it was calcined at 550°C for 2 hours, and a sample was taken to test the dispersion degree of the precious metal, which was recorded as the dispersion degree A0. Part of it was directly added with deionized water and binder, and ball milled for 15 minutes to obtain a coating solution with a solid content of 40%. The coating solution was coated on the cordierite substrate, and the remaining slurry was air-dried and roasted to test the degree of dispersion, which was recorded as the degree of dispersion B0. The cordierite substrate coated with the coating solution was dried at 120° C. for 2 h, and calcined at 550° C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com