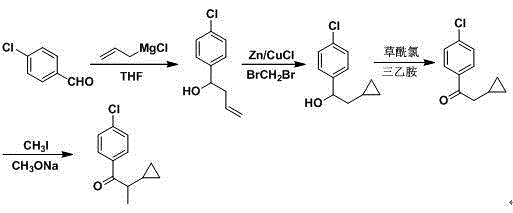
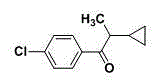
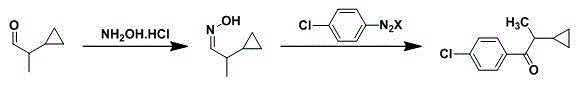Synthetic method of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone
A synthesis method and a cyclopropyl technology are applied in the synthesis field of 1--2-cyclopropyl-1-acetone, can solve the problems of low product content, high price, low total reaction yield and the like, and achieve the total reaction rate. The effect of high yield, low cost of raw materials and safe process operation
Active Publication Date: 2015-12-16
RUDONG ZHONGYI CHEM
View PDF4 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0012] The synthesis method uses reagents such as boron trifluoride and sodium borohydride, which are expensive and have certain operational risks; the total reaction yield is low and the product content is not high
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0028] 1. pH value screening
[0029] 1. Synthetic route:
[0030]
[0031] 2. Synthesis method:
[0032] Step 1: Dissolve hydroxylammonium hydrochloride in solvent ethanol, add sodium bicarbonate to adjust the pH value to 6.0; (set up four parallel conditions, see the test results in Table 1, it can be seen that the optimal pH value
[0033] is 6.0. )
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a synthetic method of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone. The synthetic method comprises steps as follows: (1), hydroxy lamine hydrochloride is dissolved in a solvent, alkali is added, the pH (potential of hydrogen) value is adjusted, a raw material of cyclopropyl propionaldehyde is added at the appropriate temperature for a reaction, and cyclopropyl acetaldoxime is obtained through extraction, concentration and drying after the reaction ends; (2), newly prepared p-chlorophenyl diazonium salt and a catalyst are added to cyclopropyl acetaldoxime at the lower temperature, acid is added, the mixture is heated to have a reaction, and 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone is obtained through aftertreatment after the reaction ends. The synthetic route is shown in the specification. The synthetic method has the advantages as follows: the cost of raw materials is low, the overall yield of the reaction is high, the technological operation is safe, aftertreatment is simple and convenient, and the synthetic method has industrial application value.
Description
technical field [0001] The invention relates to a synthetic method of a key intermediate of cyproconazole, in particular to a synthetic method of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone. Background technique [0002] Cyproconazole is a triazole fungicide developed by Sandoz (now Syngenta) in Switzerland. It has a broad bactericidal spectrum and less residue. It is a sterol demethylation inhibitor and has preventive and therapeutic effects. It is effective against powdery mildews, rusts, sporospora, coracospora, Septoria, and scabella on cereal crops, coffee, sugar beet, fruit trees and grapes. Mixed with other fungicides, it can well control cereal eye spot disease, leaf spot disease and net spot disease. At present, the sales volume on the market is increasing and the application is becoming more and more extensive, so how to mass-produce cyproconazole at low cost has become a top priority, and the key to its cost control depends on its intermediate 1-(4-chlorobenzene...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C45/51C07C49/813
CPCC07C45/516C07C249/08C07C49/813C07C251/38
Inventor 董建生叶振君袁绍志戴乙徐王正荣朱峰刘建林
Owner RUDONG ZHONGYI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com