A kind of method of solid acid catalyzed nitration p-dichlorobenzene
A technology of solid acid catalyst and p-dichlorobenzene, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., which can solve equipment corrosion, low product purity, Incomplete reaction and other problems, to achieve the effect of easy control of conditions, simplified treatment process and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 1mol of p-dichlorobenzene, heat at 70°C until p-dichlorobenzene is completely melted, then add 1g of solid acid catalyst;
[0026] Then add 1.1 mol of fuming nitric acid to carry out stirring reaction, the reaction temperature is 75-85° C., and stir for 9 hours.
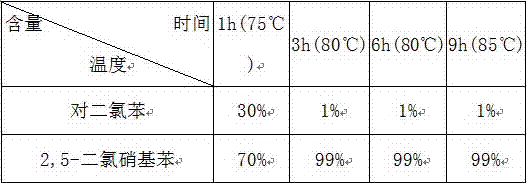
[0027] The following table is the conversion rate of p-dichlorobenzene in each time period in embodiment 1:
[0028]
[0029] Conclusion: As can be seen from the above table, the maximum conversion rate of p-dichlorobenzene is 99%.
Embodiment 2
[0031] Take 1mol of p-dichlorobenzene, heat at 70°C until p-dichlorobenzene is completely melted, then add 2g of solid acid catalyst;
[0032] Then add 1.1 mol of fuming nitric acid to carry out stirring reaction, the reaction temperature is 75-85° C., and stir for 6 hours.
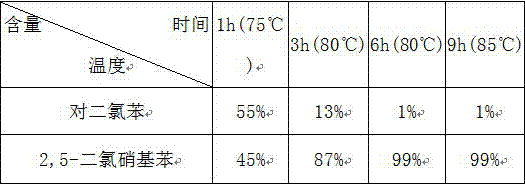
[0033] The following table is the conversion rate of p-dichlorobenzene in each time period in embodiment 1:
[0034]
[0035] Conclusion: As can be seen from the above table, the maximum conversion rate of p-dichlorobenzene is 99%.
Embodiment 3
[0037] Take 1mol of p-dichlorobenzene, heat at 70°C until p-dichlorobenzene is completely melted, and then add 3g of solid acid catalyst;
[0038] Then add 1.1 mol of fuming nitric acid to carry out stirring reaction, the reaction temperature is 75-85° C., and stir for 3 hours.
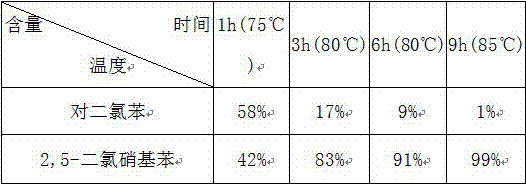
[0039] The following table is the conversion rate of p-dichlorobenzene in each time period in embodiment 1:
[0040]
[0041] Conclusion: As can be seen from the above table, the maximum conversion rate of p-dichlorobenzene is 99%.
[0042] Prepare the method for solid acid catalyst in above-mentioned embodiment 1~3, concrete steps are as follows:
[0043] (1) Prepare 0.3mol / L zirconium oxychloride solution in a PTFE beaker in a fume hood, add an appropriate amount of HF, stir thoroughly, and transfer it to a constant pressure dropping funnel after it is completely dissolved;
[0044] (2) Configure 1.0mol / L phenylphosphonic acid solution in a fume hood;
[0045] (3) Slowly add the zirconium oxyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com