Preparing method for clinic level cells for enhancing immune modulating function of MSCs
An immune regulation and cell technology, applied in the direction of animal cells, vertebrate cells, bone/connective tissue cells, etc., can solve the problems of low yield and limited immune regulation ability, achieve immune regulation and promotion, save raw material costs, and increase proliferation ability and the ability to resist NK cell killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
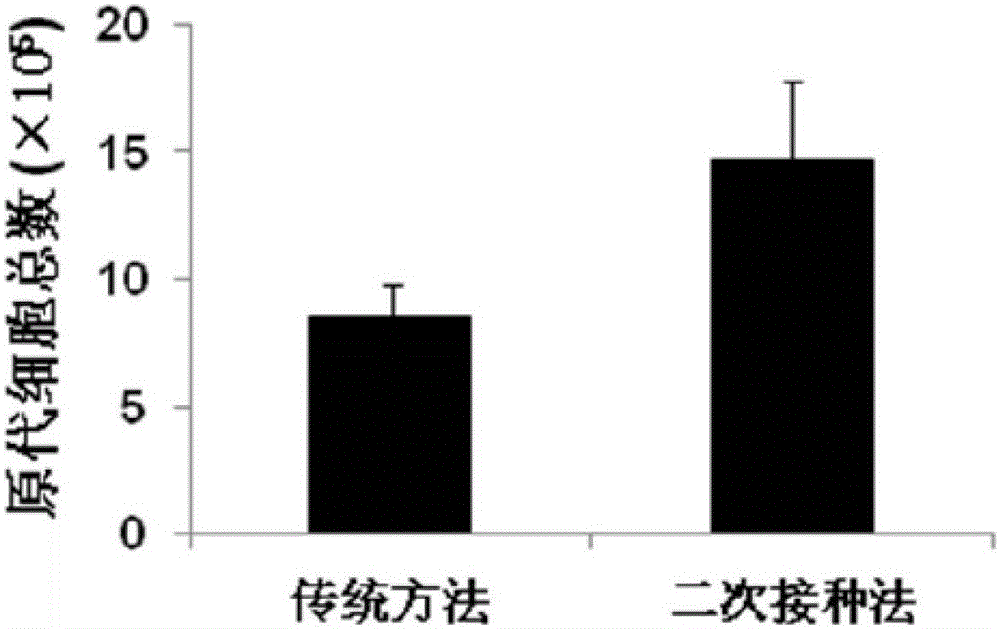
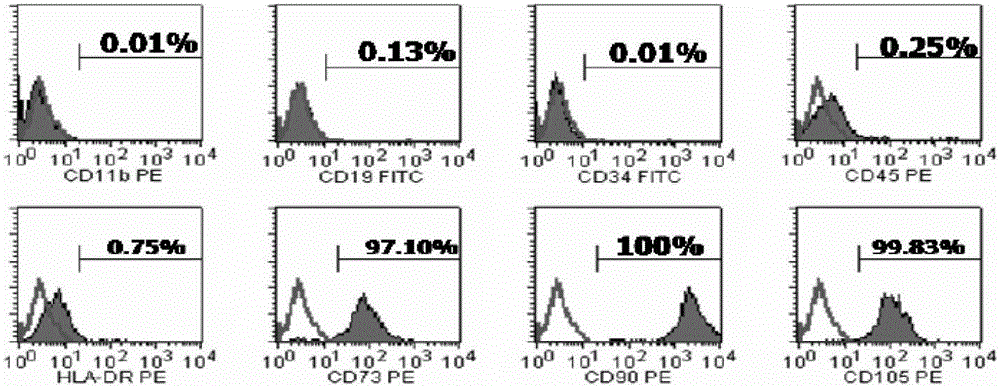
[0054] A clinical level cell preparation method for enhancing the immune regulation function of MSCs, comprising the following steps:
[0055] 1. Umbilical cord collection and mesenchymal stem cell isolation and culture
[0056] (1) Take umbilical cords of 10-15 cm from normal full-term healthy newborns, and immediately put them into DMEM medium with 10-20% mass fraction of double antibody solution; the umbilical cords need to be processed within 8-12 hours;
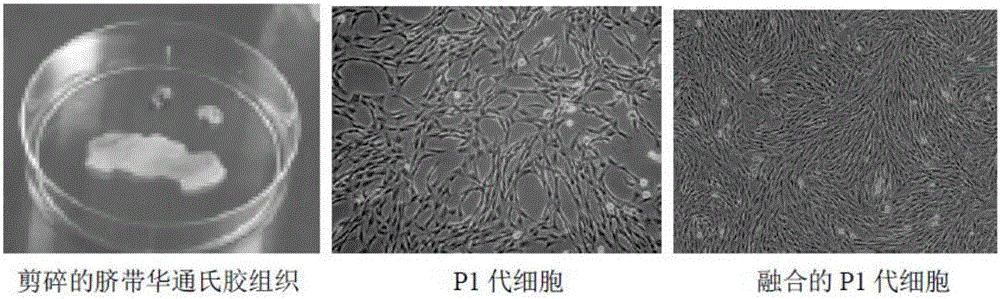
[0057] (2) Take out the umbilical cord in the ultra-clean bench, disinfect the surface of the umbilical cord with 75% alcohol, and fully wash the residual blood with normal saline; cut the umbilical cord into 1-2 cm small pieces and rinse again; cut open the umbilical cord, and draw out the umbilical vein and umbilical artery , cut the remaining Wharton's jelly umbilical cord tissue into 1-2mm 3 Size, inoculated at the bottom of T75 culture flask with an interval of 0.5-1.0 cm, and incubated the culture flask in a CO2 i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com