Battery cathode material, preparation method thereof and lithium ion battery
A battery cathode and substrate technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor cycle performance, low price, damage to the surface structure of materials, etc., and achieve the effect of good high temperature cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
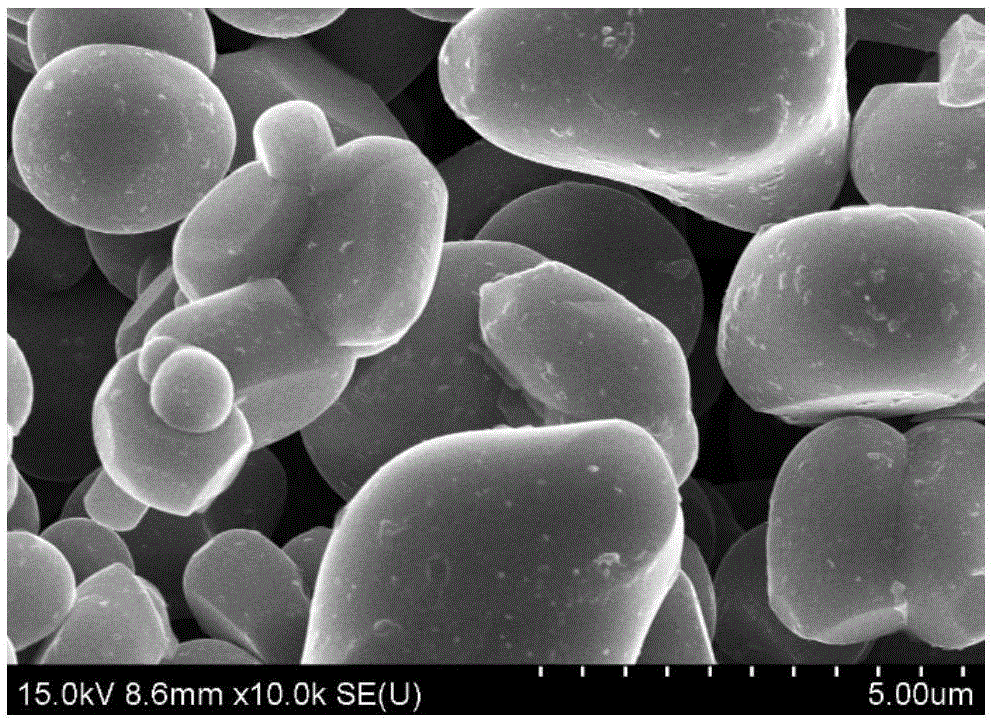
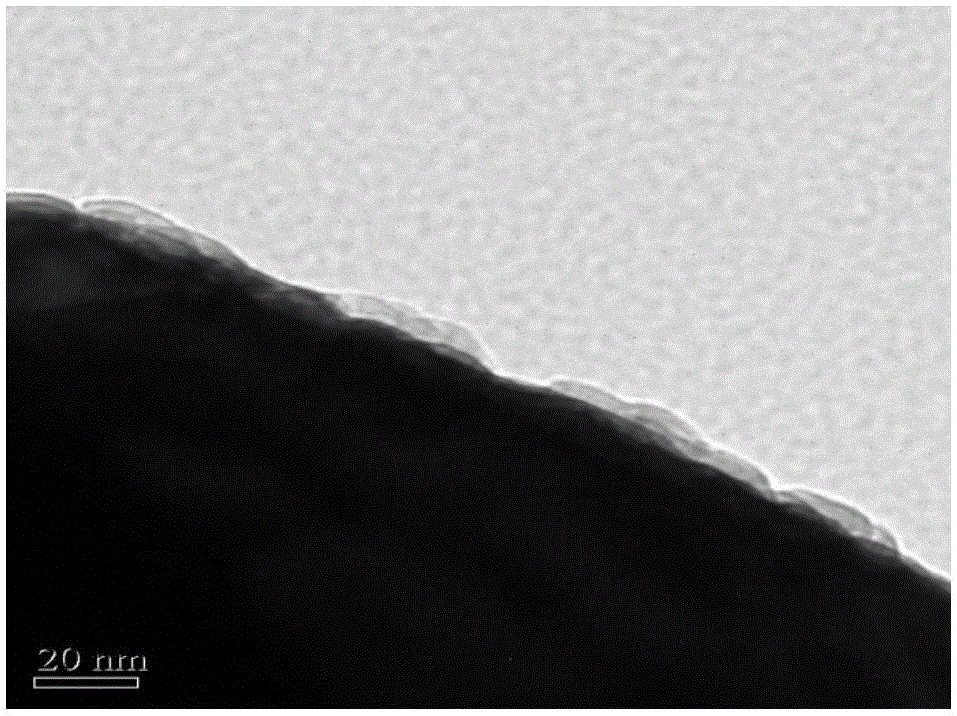
Image
Examples
preparation example Construction
[0052] The present invention provides a method for preparing the positive electrode material of a battery described in the above technical solution, comprising:
[0053] The oxide of nickel-cobalt-manganese with a single crystal structure and the lithium salt are first calcined and then kept warm to obtain an intermediate product. The temperature of the first calcination is 500°C to 800°C; the oxide of nickel-cobalt-manganese has the formula Compounds shown in II:
[0054] Ni x co y mn (1-x-y) m a o 2 Formula II;
[0055] Carrying out the second calcination of the intermediate product and then keeping it warm to obtain a substrate with a single crystal structure, the temperature of the second calcination is 801°C to 1100°C;
[0056] Described substrate is the compound shown in formula I:
[0057] LiNi x co y mn (1-x-y) m a o 2 Formula I;
[0058] In formula I and formula II, 0
[0059] M is one or more of Mg, Ti, Zr, Sr and Al;...
Embodiment 1
[0101] Dissolve cobalt sulfate, nickel sulfate, and manganese sulfate in water according to the molar ratio of Ni, Co, and Mn at a ratio of 1:1:1, and make a 1mol / L mixed solution, mix 0.1mol / L titanium nitrate aqueous solution with the above The mixed solution is fed into the reaction kettle in parallel through the respective pipelines to carry out coprecipitation reaction, and the pH value of the coprecipitation reaction is controlled to be 11.2, and the obtained coprecipitation reaction product is stirred, washed, and dried to obtain Ni with a D50 particle size of 3 μm 1 / 3 co 1 / 3 mn 1 / 3 Ti 0.01 (OH) 2 Hydroxide of nickel cobalt manganese;
[0102] Sintering the nickel-cobalt-manganese hydroxide at 600°C for 6h to obtain Ni 1 / 3 co 1 / 3 mn 1 / 3 Ti 0.01 o 2 Oxides of nickel, cobalt and manganese;
[0103] The oxide of described nickel-cobalt-manganese is ball-milled with lithium carbonate, and the consumption of the oxide of described nickel-cobalt-manganese and lithium...
Embodiment 2
[0109]Dissolve cobalt sulfate, nickel sulfate, and manganese sulfate in water according to the molar ratio of Ni, Co, and Mn at a ratio of 5:2:3 to form a mixed solution of 2 mol / L, and mix 0.1 mol / L zirconium nitrate aqueous solution with the above-mentioned The mixed solution is fed into the reaction kettle in parallel through the respective pipelines to carry out the coprecipitation reaction, and the pH value of the coprecipitation reaction is controlled to be 11. The obtained coprecipitation reaction product is stirred, washed and dried to obtain Ni 0.5 co 0.2 mn 0.3 Zr 0.01 (OH) 2 Hydroxide of nickel cobalt manganese;
[0110] Sintering the nickel-cobalt-manganese hydroxide at 600°C for 6h to obtain Ni 0.5 co 0.2 mn 0.3 Zr 0.01 o 2 Oxides of nickel, cobalt and manganese;
[0111] The oxide of described nickel-cobalt-manganese and lithium carbonate are ball-milled and mixed, and the consumption of the oxide of described nickel-cobalt-manganese and lithium carbonat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com