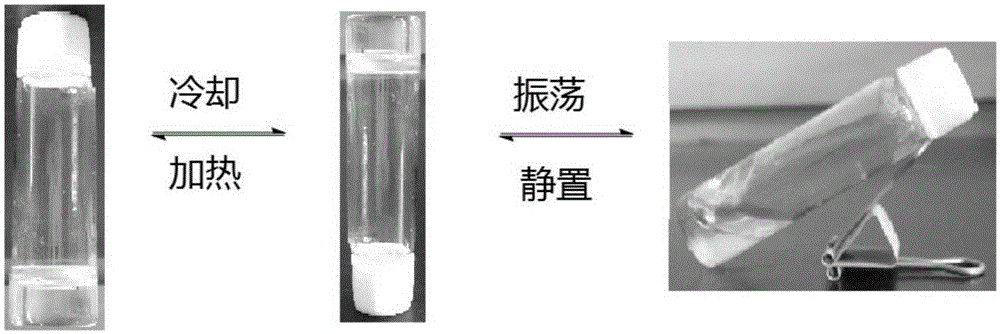
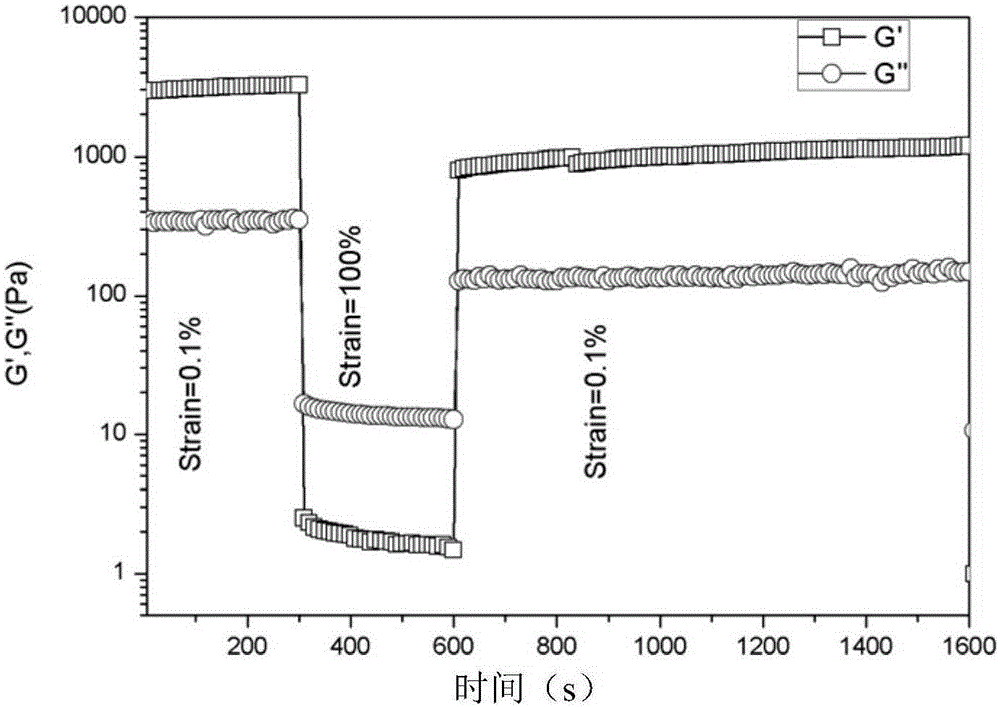
N-amino alkyl substituted glucosamide as well as preparation method and application thereof
A technology of glucosamide and amine alkyl, which is applied in the field of N-aminoalkyl substituted glucosamide and its preparation, can solve the problems of long recovery time and low instantaneous recovery rate, and achieve high instantaneous recovery rate and reversible thixotropic process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Raw material 2,4-(3,4-dichlorobenzylidene)-D-methyl gluconate (II) used in the present invention is made by following method:
[0035]
[0036] Add 215.8 g of 50 wt % D-gluconic acid aqueous solution (0.55 mol of D-gluconic acid), 100 mL of methanol, and 200 mL of concentrated hydrochloric acid into a 1L four-necked flask equipped with mechanical stirring and a thermometer at room temperature, and stir at 200 rpm. Add a methanol solution of 3,4-dichlorobenzaldehyde (dissolve 87.5g (0.50mol) of 3,4-dichlorobenzaldehyde in 300mL of methanol), and the system becomes viscous after 4 hours of reaction. Minute speed stirring reaction for 20h, after the reaction is completed, add 100mL water to the system, stir for 2h and then filter with suction, wash the filter cake with a large amount of water until the pH is 6-7, then wash twice with 200mL of hot dichloromethane, and drain to obtain Product II, dried to yield 140 g. The yield was 76%, and the melting point was 188.6-18...
Embodiment 2
[0038] The preparation method of N-aminoalkyl substituted glucamide comprises the steps:
[0039] With 4-dimethylaminopyridine (DMAP) as catalyst, 2,4-(3,4-dichlorobenzylidene)-D-gluconate methyl ester (II) and aliphatic diamine (NH 2 C n h 2n NH 2 ) is a raw material, and pyridine or methanol is a synthesized N-aminoalkyl-substituted glucose amide molecule (I) under the condition of a solvent, and the reaction equation is as follows.
[0040]
[0041] Said n=2, 4, 6, 8 or 12.
Embodiment 3
[0043] N-(2-aminoethyl)-2,4-(3,4-dichlorobenzylidene)-D-glucamide (G 2 ) preparation method:
[0044] Add 5.00g (0.014mol) 2,4-(3,4-dichlorobenzylidene)-D-gluconic acid methyl ester (II) to a 250mL four-neck flask equipped with a mechanical stirrer and a thermometer at 10°C , 50 mL of pyridine was used as the solvent, and 0.01 g (0.008 mmol) of DMAP was used as the catalyst. After stirring for 30 min, 2.52 g (0.042 mol) of 1,2-ethylenediamine was added and stirred overnight at room temperature. After the reaction was completed, 25 mL of water was added to the system, stirred for 2 hours, then filtered with suction, and the filter cake was washed with water, then washed with methanol and dried to obtain a crude product. The crude product was refluxed in 20 mL of methanol for 30 min, cooled to room temperature, stirred for 1 h and then filtered with suction. The filter cake was washed with methanol and dried to obtain 3.9 g. Compound N-(2-aminoethyl)-2,4-(3,4-dichlorobenzylide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com