Enzymatic preparation method of andrographolide glucoside derivative
A technology for andrographolide and enzymatic preparation, applied in the direction of fermentation, can solve the problems of environmental pollution, poor selectivity, high energy consumption, etc., and achieve the effects of simple and easily controllable reaction process, mild reaction conditions, and easy separation of products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
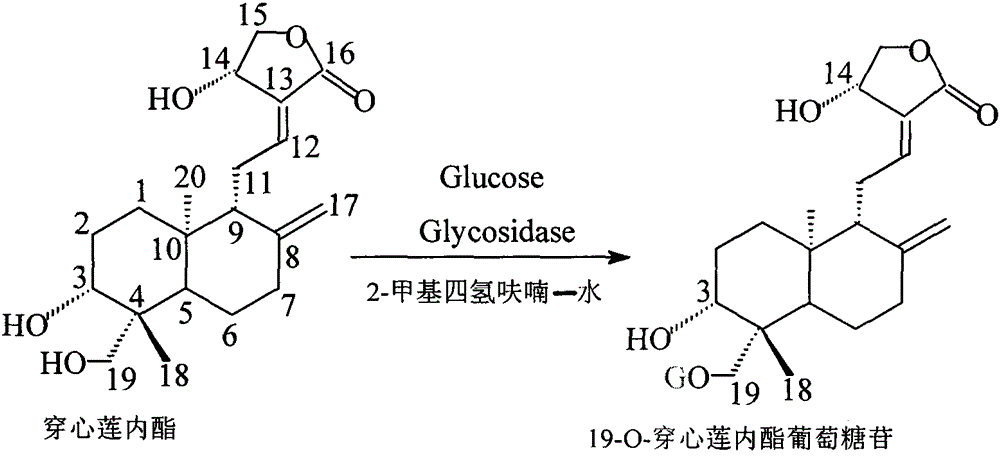
[0015] Put 1g of andrographolide, 3g of glucose, 1g of immobilized β-glucosidase (250U / g) from almond and 25ml of 2-methyltetrahydrofuran-water (80:20) into a stoppered Erlenmeyer flask at 30 ℃, 100rpm in a constant temperature oscillator, reacted for 24h, filtered to remove the immobilized glycosidase, then evaporated to remove the solvent under reduced pressure, the resulting mixture was dissolved in methanol, and then purified with a C18 column, the eluent was dichloromethane: Methanol: water = 30: 20: 10, then concentrated and vacuum-dried to obtain a white powder which is 19-O-glucose-andrographolide glycoside derivative (yield 25.5%).
Embodiment 2
[0017] Put 1g of andrographolide, 3g of glucose, 2g of immobilized β-glucosidase (250U / g) from almond and 25ml of 2-methyltetrahydrofuran-water (90:10) into a stoppered Erlenmeyer flask at 35 ℃, 100rpm constant temperature shaker, after 36 hours of reaction, filter to remove the immobilized glycosidase, then evaporate to remove the solvent under reduced pressure, the resulting mixture is dissolved in methanol, and then purified with a C18 column, the eluent is dichloromethane: Methanol: water = 30: 20: 10, then concentrated and vacuum-dried to obtain a white powder which is 19-O-glucose-andrographolide glycoside derivative (yield 37.3%).
Embodiment 3
[0019] Put 1g andrographolide, 3g glucose, 3g immobilized β-glucosidase (250U / g) from almond and 25ml 2-methyltetrahydrofuran-water (90:10) into a stoppered Erlenmeyer flask, place at 40 ℃, 100rpm constant temperature oscillator, after 72 hours of reaction, filter to remove the immobilized glycosidase, then evaporate the solvent under reduced pressure, and dissolve the resulting mixture with methanol, then purify it with a C18 column, and the eluent is dichloromethane: Methanol: water = 30: 20: 10, then concentrated and vacuum-dried to obtain a white powder which is 19-O-glucose-andrographolide glycoside derivative (yield 67.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com