Ambroxol hydrochloride oral solution and preparation method thereof
A technology for oral administration and solution of ambroxol hydrochloride, which is applied in the field of oral ambroxol hydrochloride solution and its preparation, can solve the problems of unstable dosage form of oral solution, affecting patients' willingness to use, hidden dangers of drug safety, etc., and achieves extended shelf life and enhanced use Willingness, the effect of stable production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
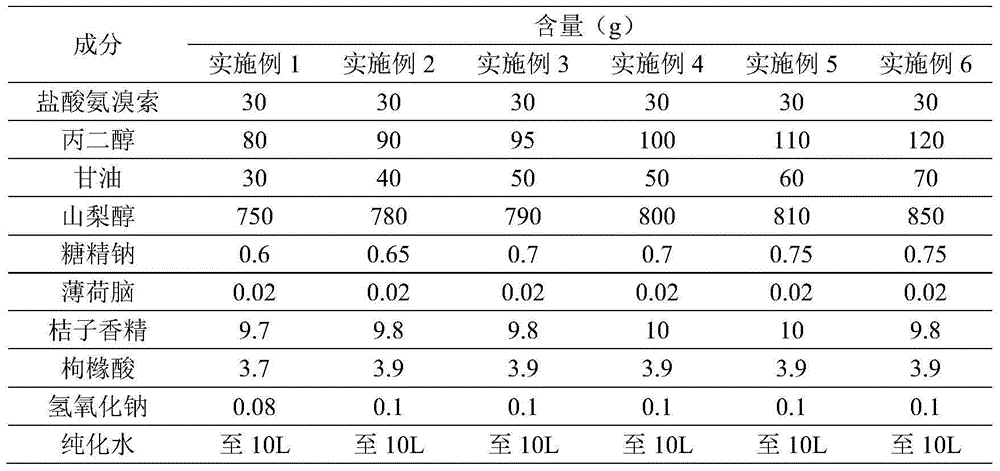
[0032] Prepare ambroxol hydrochloride oral solution according to the ratio of raw materials in the following table 1:
[0033] Table 1, ambroxol hydrochloride oral solution raw material proportioning
[0034]
[0035] The preparation method is as follows:
[0036] 1) Pretreatment of raw and auxiliary materials
[0037] Grind the menthol and sodium saccharin and pass through a 60-mesh sieve for later use;
[0038] 2) Dosing
[0039] a. Add menthol to 50°C pure water, stir and dissolve to make a 3 mg / ml menthol solution; add sodium hydroxide to pure water to prepare a sodium hydroxide solution;
[0040] b. Add propylene glycol to purified water, reflux and stir for 2min; then add ambroxol hydrochloride and reflux and stir for 10min until completely dissolved; Glycerin, menthol solution and orange essence were respectively refluxed and stirred for 2 minutes until they were evenly mixed;
[0041] c. Adjust the pH with sodium hydroxide solution, make up to volume with purif...
Embodiment 7
[0048] 1. Stability testing
[0049] One group of ambroxol hydrochloride oral solution is randomly selected in embodiment 1-6 and carries out following inspection successively, and testing result is as follows:
[0050] 1) Influencing factor detection data:
[0051] a) Light factor
[0052]
[0053] b) Temperature 40°C
[0054]
[0055] c) Temperature 60°C
[0056]
[0057] 2) Low temperature freeze-thaw cycle detection
[0058] a) Low temperature cycle test data
[0059] Investigation conditions: including three cycles, each cycle should be at 4°C for 2 days, and then at 40°C for 2 days under accelerated conditions.
[0060]
[0061] b) Freeze-thaw cycle detection data
[0062] Investigation conditions: Including three cycles, each cycle should be under the condition of -10--20°C for 2 days, and then under the accelerated condition of 40°C for 2 days.
[0063]
[0064] 3) Accelerated detection
[0065]
[0066] 4) Long-term detection
[0067]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com